13. QSE Facilities and Safety Management
According to the CLSI, the “QSE Facilities and Safety Management provides information about the laboratory’s physical environment and maintenance and safety programs needed to support it”.[1]In other words, this QSE provides the information to tell us how to look after the laboratory to keep the physical building running smoothly and safely. We will look at the policies, processes and procedures that support the day to day protection of the physical space.
Policies that are part of this QSE that apply to Facilities state intent and direction for
- Facility design and modification or renovation
- Facility access
- Uses of the facility and maintenance requirements
- Communication system for the facility
The processes that transform these intents into action include processes
- For the allocation of space, facility design and modification
- For determining who should have access to the facility and how access is gained
- For facility maintenance and monitoring environmental conditions
- For communication throughout the facility
The procedures that need to be in place to provide documented instructions to support these processes include procedures for
- Determining how space requirements and needs are determined
- Evaluating how design and renovation plans are for efficiency, comfort, accommodations of people with disabilities, workflow and emergency systems
- Planning for adequate lighting, energy sources, water, waste disposal, and utilities
- For use of access control devices
- For maintaining clean work areas
- For monitoring, controlling, and recording environmental conditions that could impact the quality of test results
- For communication devices such as telephones, pagers, buzzers, email, etc and for emergency contact within the facility
Safety programs also have policies, processes and procedures that support the effective and efficient functioning of the facility.
Policies include those that address intent and direction for
- Biosafety
- Chemical hygiene
- Occupational health – accidents, illnesses and exposures
- Hazardous waste
- Fire prevention
- Emergency preparedness, response and recovery
- Radiation safety
Processes that transform the intent into action include those for
- Exposure control plans and Infection control training programs for blood and air borne pathogens
- Evaluation process to evaluate the effectiveness of such plans
- Transportation of potential biohazardous materials
- Chemical hygiene plan and training program
- Annual review of the effectiveness of the chemical hygiene program
- Management of laboratory accidents
- Monitoring and mitigating noise levels
- Hazardous waste disposal plan and training program
- Fire prevention and control plan and training programs for fire extinguisher use
- Internal and external preparedness plans, evacuation plans, and training programs
- Radiation safety plan and training program
There are also procedures that must be put in place to give documented instructions for all these processes including those for
- Use of PPE
- Collecting blood specimens from patients with special precautions
- Cleaning up biological spills and chemical spills
- Preparing hazardous materials for shipping, both biological and chemical
- Managing WHMIS
- Reporting laboratory accidents and laboratory-acquired illnesses
- Identifying the waste stream disposal procedures for various hazardous wastes
- Reporting fires, taking follow-up action, and recording staff participation
- Assigning roles and responsibilities for declared disasters, evacuation procedures, conducting and recording evacuation drills
- Using radiation badges properly
- Posting radiation signage
- Monitoring radiation and taking action when acceptable levels are exceeded[2]
Facility Design and Modifications
Laboratory management needs to work with the facility personnel and often with external design specialists when building a new laboratory or when contemplating making a renovation to an existing structure. There are a number of factors that must be taken under consideration in any design features:
- Space allocation – there must be enough space to accommodate the required equipment and analyzers as well as sufficient room for staff to maneuver around the space safely. Since it is important to maintain confidentiality for patients and their private information, there must also be space for confidential conversations, access for disabled persons, area to allow for comfortable blood collection, and room to protect visitors and staff from coming into contact with unnecessary workplace hazards.
- Environmental factors – the design must accommodate environmental requirements such as lighting, ventilation, water, heat, and waste disposal. In order to meet these requirements, there must be a clear understanding of how the future space is going to be used and what equipment and analyzers will be functioning in the space. Many analyzers give off significant amounts of heat so the ventilation system must be able to keep up with the output. Otherwise, the analyzers may shut down and not function. The working conditions may also become unbearable for staff if the room becomes too hot. The placement of analyzers may also be impacted on where the electrical outlets are located and how close they are to waste disposal systems and sources of water.

Biohazard Buckets waiting for pick up, Waterfront MLT Laboratory. Credit: NSCC / W. Bryan. - Storage requirements – depending on the type, size and volume of various items for storage, there are a number of different considerations that must be taken into account. Some examples of the types of things that may to be stored either within the space, or externally include:
- Samples – need to be refrigerated, frozen or kept at room temperature, depending on the type of specimen and the tests requested on it
- Slides and Histology blocks – need to meet environmental conditions that will allow them to maintain the integrity of the articles and keep them from shriveling up, drying or cracking
- Blood products – require controlled temperature refrigeration
- Retained microorganisms – must be stored at minus 70 degrees in either glycerin or skim milk to avoid crystals forming that could harm the organism.
- Reagents and laboratory supplies – must be stored under controlled temperatures that may be room temperature, refrigerated or frozen at minus 20 degrees. For long term storage, they may require storage at minus 70 degrees to reduce degradation of the materials.
- Equipment storage – requires a dust free environment and controlled temperatures. They also may require protection from being smashed, bumped, or damaged by other articles moving about the space
- Documents, records, files and manuals – require storage, sometimes for long periods depending on the information contained in them. Some items must be stored near at hand where they are easily accessible but others may actually be stored off-site at remote long-term storage locations.

Chemical storage at Waterfront MLT Laboratory. Credit: NSCC / W. Bryan.
- Access – controlled access must be maintained throughout the laboratory to protect the staff, specimens and supplies from unwanted intrusions.Specimens must be secure from the threat of tampering and supplies must be kept safe from theft, tampering, or other misuses. Staff members, on the other hand, have security access only to areas of the laboratory where they work. They do not need access to areas where they don’t work for their own protection from foreign sources of infection or injury because they are not adhering to the appropriate personal protective apparel requirements or because they are not aware of other potential hazards.

Bio-hazard signs are placed on all entry doors into each MLT lab at Waterfront campus. Credit: NSCC / W. Bryan. - Communication – the facility requires a means of communicating with all personnel in a timely manner in the event of an emergency. Fire bells that have different signals for different conditions are used in many organizations and email is used in situations where it is important to get a universal message to all employees, although it may not be urgent for everyone to hear it for safety reasons. Email is also used to communicate when details of the emergency are needed for ongoing response. In some laboratories, a loudspeaker system that carries through the facility may be used to transmit additional information as well.
Discussion points
- Think about the layout of an Outpatient Blood Collection centre – what could happen if the phlebotomy draws weren’t separated from the waiting area – from the perspectives of confidentiality, privacy, and safety?
- And what about the Histology lab – how would the staff function if the grossing area and processing equipment were right beside one another? And what about staining? How effective would the lab run with everything crowded together in one small room from an environmental perspective?
Safety programs
Medical Laboratory staff are well trained in Safety practices for clinical laboratories. The programs covered in the CLSI documents are the same ones that students learn about in previous courses, although the CSMLS Safety Guidelines give more detailed information about the specifics of safe practice that Medical Laboratory Professionals must abide by to maintain a safe work environment.
- Biosafety – refers to the plans laboratories must put in place to address biohazard exposure, whether through working with live organisms, blood specimens, or infected patients. They include such items as hepatitis vaccination programs, monitoring and follow-up procedures for blood borne pathogens and TB, spill containment procedures, procedures for the prevention and monitoring of needle stick and sharps injuries, procedures for preparing hazardous specimens for shipping, and training programs for the various aspects of biosafety. Can you think of any other biosafety issues that need to be addressed in the clinical lab?
- Chemical Hygiene and Hazard Communication – refers to the handling and storage of hazardous chemicals in the clinical laboratory. This includes such procedures as those for hazard evaluation in the workplace, spill and containment procedures for all chemicals found in the laboratory, the identification and labeling of all chemical containers, developing and maintaining a library of MSDS for all chemicals housed in the laboratory, and a process for the annual review of the chemical hygiene process to ensure that all required protective measures are in place.
- Hazardous Waste Management – refers to the disposal and record keeping for all hazardous waste from the facility. It includes a process for sorting and storing the waste while it awaits disposal as well as procedures for staff training. The Hazardous Waste program must be compliant with all municipal, provincial and national regulations that are in effect in the jurisdiction.
- Fire Prevention – refers to the programs and procedures that must be in place to support fire prevention within the laboratory and to ensure that all staff are trained in the fire codes, use of extinguishers, and evacuation procedures for that facility.
- Emergency Measures management – refers to the processes and procedures that are in place to address emergency situations as they arise. Examples of these types of situations would include
- First aid for cuts, burns, spills, and other similar types of events
- Handling fires and electrical equipment malfunctions
- Evacuation plans
- Staff list for emergency contact
- Roles and responsibilities in the case of emergency
- Response for external disasters that could impact laboratory operations, either by requiring a heightened level of laboratory response, or for enabling staff to get to and/or from work effectively and safely
- Radiation Safety – refers to the need for a safety program to address risk assessment and protection of staff who may use radioactive materials in the course of their day to day work. The amount of radiation present in the clinical laboratory has been drastically reduced in recent years but there are still some applications where radiation is present.
- Safety Training – refers to the training required for all staff to be able to work safely and securely within the laboratory environment.
Can you list some of the areas where laboratory staff must all receive training in safe practice in the Clinical Laboratory?

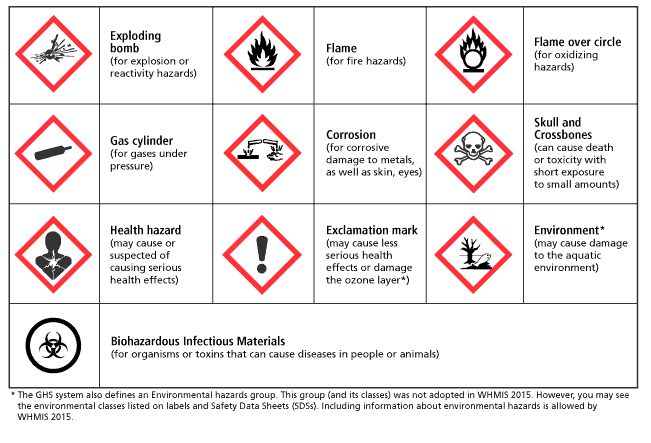
How many of these hazard symbols can you identify?
Review Questions
- Which of the following types of waste would NEVER be disposed of in a box?
- Liquid waste
- Autoclaved biohazardous materials
- Chemically contaminated glassware or plastic ware
- Chemical containers that cannot go in regular waste
- What is one example of a process/procedure from the Emergency Measures Management?
- A hazard evaluation
- An evacuation plan
- A Vaccination program
- Transportation of Dangerous Goods legislation
- Which of the following pieces of information is NOT required on an MSDS?
- Preventative measures
- Protective clothing
- Preparation information
- Supplier’s name
- What are the 6 areas of consideration for Facilities planning?
- What are the 7 areas that must be addressed for Safety in a laboratory?
