1. Introduction
Historical Background
Quality Management Systems (QMS) in healthcare play a major role in efforts to improve patient care. Putting quality measures in place helps to protect patients from errors in treatment and diagnosis, as well as to protect them from institutionally acquired infections and diseases. Patient safety became a major focus in the late 1990’s when the Institute of Medicine in the USA published a report called To Err is Human: Building a Safer Health System. This report describes the serious issues facing patients who enter the health system looking for help and some of the obstacles that they encounter. The report contains many recommendations for improving patient care by introducing patient safety measures that would allow patients to receive the care they need without fear or risk of injury. The book was the first in a series of reports that are designed to help develop strategies to improve the overall quality of patient care.
Julie Hammerling[1] describes some of the improvements that have taken place over the past number of years to reduce errors in laboratory services. According to Hammerling, “while many areas of health care are still struggling with the issue of patient safety, laboratory diagnostics has always been a forerunner in pursuing this issue. The concepts and practices of quality assessment programs have long been routine in laboratory medicine, and error rates in laboratory activities are far lower than those seen in overall health care”.[2] She describes how analytical errors have been controlled by using external quality assessments, or proficiency testing programs, which have been used to detect trends or bias, to investigate root causes of problems when results are unacceptable, and to verify the effectiveness of problem resolution in clinical decision making.
Some of the processes that have been developed to help reduce pre-analytical errors include:
- Ensuring the development of clearly written procedures
- Enhancing health care professional training to include learning about QMS and its impact on patient safety
- Automating functions, such as bar code reading and order entry
- Developing and ongoing monitoring of quality indicators
- Developing improved lines of communication among the various health professionals and fostering interprofessional collaboration
Post-analytical errors have been reduced by automating the reporting function and improving communication between the laboratory and the clinical staff. Improvements have been achieved through use of the laboratory information system (LIS) integrating with other management information systems. Reports are received electronically as soon as they are completed instead of having to be sent through the mail and taking 2-3 days to reach the physician. Timelier reporting of results has in turn led to improved diagnosis and treatment times for patients. Lastly, Hammerling identifies that incident, or non-conformance, reporting has been the focus of much of the improvement seen in medical laboratories. Through tracking errors, performing root cause analysis, and standardizing process throughout the lab, many potential errors have been corrected or avoided completely.
Read the Full Article
A Review of Medical Errors in Laboratory Diagnostics and Where We Are Today by Julie A. Hammerling published in Laboratory Medicine. February 2012.
Quality processes in laboratory services were first introduced many years ago with the implementation of quality control( QC) which was already being widely used in manufacturing and other industries. This implementation was brought about by news of preventable deaths, as well as errors in diagnosis and treatment of patients. It became clear that better processes for performing lab testing needed to be found and implemented. QC provides a means to monitor precision and accuracy in lab testing. The use of QC was followed in the late 1980s and early 1990s by the introduction of quality assurance (QA). QA is the process by which laboratory personnel start to look at how services are delivered and how these services could be improved to provide a higher quality, safer level of service. Quality Improvement (QI) initiatives were implemented to either improve the current service or to correct problems in the way service was delivered. By the year 2000, healthcare facilities around the world had started to take an even broader approach to quality by introducing Quality Management Systems (QMS) as a response to the report on patient safety that was released by the Institute of Medicine in the USA.
The purpose of the quality management system is to provide a platform for continuous improvement and to enhance the outcomes of patient care.
The QMS helps to:
- Reduce or eliminate medical error
- Increase to the ability to meet patient requirements
- Provide a means to implement successful means of accrediting assessment
- Sustain the attainment of quality objectives.
The Current Canadian Model
The model for quality management systems that is most commonly utilized in Canada today is the model that was introduced by the Clinical and Laboratory Standards Institute (CLSI) as an approved guideline in 2001, was further developed in the approved guideline of 2004, and again most recently reviewed and revised in 2019: A Quality Management System Model for Laboratory Services: Approved Guideline, 5th edition. According to CLSI, “the goal of an efficient and effective laboratory is to consistently provide the appropriate examinations with accurate results in a timely manner and with the most judicious use of resources.” [3]This model is based on the ISO 15189:2012 (most recent updated version) standards, including some aspects of ISO 17025 whose focus is on both management and technical operations of testing and calibration labs.
ISO 15189:2012 defines the requirements for both quality management and technical operations for medical laboratories. Then International Organization for Standardization (ISO) is the world’s largest nongovernmental developer and publisher of international standards. Currently 69 countries around the globe use the ISO15189 standards, either the original version or as a basis for developing their own national standards. According to the ISO 15189:2012 Handbook, “medical laboratory activity is directly linked to patient safety and the impacts of medical error. Laboratory results that are invalidated because they are wrong or misleading can lead to wrong diagnosis or treatment error and sometimes have catastrophic results” [4]
Question
- What are some examples of outcomes that could happen if the physician acted on the incorrect test results?
QMS key Elements
The key elements of the QMS are described as the basic requirements that are needed to make sure that the organization’s policies, processes and procedures are carried out successfully. Key quality elements include the quality system essentials, the quality manual, the path of workflow, and the policies, processes and procedures that are used to describe the work of the organization in the quality system essentials. These key elements are the building blocks needed to build a firm foundation for the QMS.
The 12 quality system essentials (QSEs) are listed below:
- Organization and Leadership
- Customer Focus
- Facilities and Safety Management
- Personnel Management
- Supplier and Inventory Management
- Equipment Management
- Process Management
- Documents and Records Management
- Information Management
- Nonconforming Event Management
- Assessments
- Continual Improvement
Over the course of this semester, we will be learning about the 12 QSEs and how they fit together to provide an effective model for building a quality management system. All of the QSEs must be in place and functioning effectively so that the work of the organization can be carried out to produce a high quality set of operations. In laboratory services, these operations result in the provision of accurate and timely production of patient test results.
Quality Manual
The Quality Manual describes what the quality management system is and contains all of the policies that define the work of the laboratory for the organization’s personnel, customers, and external assessors. It lays out who is responsible for the development and review of each policy, lists the policies that govern each of the twelve QSEs, details the scope of each policy and who it is done for, and finally describes the scope of the organization’s QMS.
The cornerstones of the Quality Manual include:
- Communication to personnel, customers, and external assessors
- Provides reference to the organization’s policies, processes, and procedures
- Defines how the organization functions to meet the identified requirements.
- Serves as procedure manual for management (p.16)
The Quality Manual will be discussed in more detail under the QSE Organization and Leadership.
QMS documents
The last component of the key elements is the QMS documents. We will talk in detail about these documents when we discussion the QSE for Documents and Records Management but for now, it is important to know that the documents of the QMS consist of policies, processes and procedures. Do you know what the difference is between a policy, a process and a procedure?
According to the CLSI, “policies are statements of the organization’s intentions or commitments and answer the question what do we do? “A Quality policy states the organization or service’s intent regarding the QSEs”[5]
QMS Processes describe the activities that must be completed to fulfil the requirements of each policy. For each policy, there may be several processes required. Processes describe the activities that need to be completed to accomplish the intent of the policies, the correct sequencing of the activities to successfully complete the process, and the person who is responsible for each process. There are several processes included in one policy and most often processes are depicted as flowcharts or tables to make easy reference to the order of the procedures that will be performed.
Think about Blood Collection
What processes do you think would be needed to fulfil the requirements of a policy that stated “We will provide high quality blood collection services for both inpatients and outpatients that will allow the testing to be completed in an efficient, timely manner to support the highest level of patient care” ?
QMS procedures
QMS procedures list the step-by-step procedures that must be followed in order to complete a particular part of the process. There may be several procedures needed to address one process. For example, the process for collecting blood specimens in the outpatient clinical would include procedures for each of the following activities:
- registering patients into the LIS
- calling patients into the phlebotomy area to have the collection performed
- performing the phlebotomy procedure
- transporting the specimens from the collection area to the specimen processing work area.
Each procedure must be developed, reviewed and approved. Once they are approved, the staff working in the outpatient clinic would be informed of the procedure and then trained to follow it accurately. It is not of much use to staff to have a process developed if it is not clearly articulated to all staff so that the relevant people can receive the proper training.
Sample flowchart for the process of Blood Collection Services
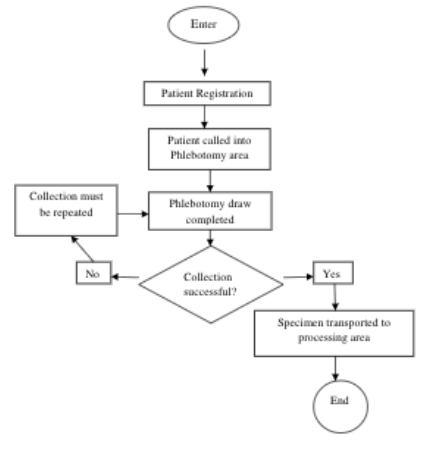
The Path of Workflow Concept
The Path of Workflow is made up of processes from the pre-examination (pre-analytical), examination (analytical), and post-examination (post-analytical) phases that are carried out in the laboratory. The following table shows some of the key categories in the Path of Workflow and where they are placed.
| Pre-examination | Examination | Post –examination |
|---|---|---|
| Ordering examinations | Method selection | Preliminary reports |
| Sample collection | Examination performance | Final reports |
| Sample transport | Review of results | Sample management |
| Sample processing | Interpretation |
Each of these categories are made up of several processes and activities. For example, ordering examination would include proper identification of the patient, along with any necessary patient information such as time medication was administered and the dosage given, date of birth, status of the testing request, and special instructions. The examination performance would include following the documented procedure for the examination being performed, ensuring the technologist is competent to perform the procedure and that it can be performed at an available work station. Review of results would require that the quality control was properly verified to ensure it is working effectively and that trouble shooting activities have been documented and approved.
Other important aspects of the path of workflow include ensuring that staff training and competency assessment programs are documented and have written policies, processes and procedures to guide their performance. The same is true for work processes relating to equipment purchase, maintenance, and service. All the documented policies, processes and procedures must be developed and followed in a standardized manner in order to ensure accurate, timely testing is completed. As you continue through the study of the 12 QSEs, keep in mind that they all fit into the path of workflow and must be appropriately set up to support an effectively operating QMS. Having these supports in place is an important part of ensuring that the laboratory is able to meet its accreditation requirements.
There are also a couple of other concepts that should also be taken into consideration when developing a Quality Management System in a clinical laboratory.
Ethics in healthcare
Ethics in healthcare as it pertains to the QMS is described as doing “good” and not doing “harm” to the patients we serve. Included in this topic are issues that deal with moral conduct, duty, and personal judgement of employees and the employer. In Canada, ethics are imbedded in the Code of Professional Conduct for the Medical Laboratory Technologist and each employee is expected to act within that code.
Question
- Are you familiar with the CSMLS Code of Professional Conduct for Medical Laboratory Technologists? What does it include?
Ethical behaviors are applied to personnel in the laboratory and they are also applied to the testing performed in the lab. When describing the ethical behavior of personnel, CLSI describes ethical behavior as “issues of undue internal or external commercial, financial, or other pressures and influences that could adversely affect the quality of their work” (p.14). When describing ethical behavior in relation to testing, CLSI says “in maintaining a relationship of confidence and trust with customers, ethical practices in the activities conducted during the pre-examination, examination, and post-examination phases and the QSEs are critical” (p.14). That means that as employees and as an organization medical laboratory technologists are required to maintain strict confidentiality of all patient information, safeguard the privacy and dignity of all patients, always deal with vendors and lab suppliers at arms-length, and never misrepresent their role on the healthcare team. All patients should be treated fairly and without discrimination.
One way laboratory personnel can act in an unethical manner is to develop a relationship with a vendor or supplier where the technologist will benefit by receiving funding to go to a conference or seminar, receive a gift at Christmas time, or accept some other benefit. Those are just a few examples of things that are considered to be unacceptable in the eyes of the organization, the profession and the general public. Laboratory technologists are also responsible for the quality of the test results produced by their work and must ensure that all patient samples, including blood, tissue, body fluids, etc. are treated with respect.
According to the ISO1589:2007 Handbook, there are 8 areas where ethical behaviour is a prime consideration[6]:
- Collection of Information from/ about patients – adequate information should be collected but not unnecessary personal information. Patients should always be informed of why the information is necessary and what it will be used for.
- Collection of Specimens – informed consent should always be obtained, either direct or implied. Patients must always be given the option of refusing to have the specimen collected and adequate privacy must be provided. If a specimen is determined to be unsuitable by the laboratory, the specimen must be discarded and the ordering physician informed.
- Performance of testing – all testing must be carried out by competent professionals and fabrication of any test results is unacceptable.
- Reporting of Results – all results must be reported accurately, and those of a specified patient must remain confidential and can be reported to the ordering physician only. Results may be reported to other individuals with the patient’s consent or when required by law. All patient identification must be removed before the results can be used for purposes such as epidemiology, demography, or statistical analysis.
- Storage and Retention of Medical Records – all information must be stored safely to guard against loss, unauthorized tampering or other misuses in accordance with legislative requirements.
- Access to Medical Laboratory Records – access to patient records would normally be limited to laboratory staff or the originating physician. There may be exceptions in accordance with legal authorization.
- Use of Samples for Purposes other than Those Requested – Use of samples for research or educational purposes should only be used either with the patient’s consent or after all identifying information is removed.
- Financial Information – medical laboratories should not enter into financial agreements with physicians or funding agencies where the agreement provides an inducement to perform examinations or interferes with the requesting physician’s assessment of what’s in the best interest of the patient or where there may be a real or perceived conflict of interest.
Exercises
Read the following statements. Do you agree or disagree that these describe good ethical practice? Why or why not?
- Do not release results of questionable quality.
- It is alright to discuss a patient’s condition with the family of the patient if you know them.
- Do not cheat on proficiency testing surveys.
- Do not accept gifts of significant value from vendors.
- Report a lost specimen as “laboratory accident” instead of “lost specimen”.
- Perform only those tests requested.
- Lab employees should not criticize the organization.
- Do not come to work under the influence of Alcohol or drugs.
The Cost of Quality
What do we mean by “the cost of quality”? When we are learning about quality, the costs associated with providing high quality services must be taken into consideration. Quality does not come without a cost but the overreaching goal for any organization is that the cost of providing quality services is outweighed by the cost of bad service so that building, implementing and maintaining a QMS becomes a cost saving endeavour.
The cost of providing a quality management system includes a number of factors. Participating in proficiency testing is one major cost. The lab must join a recognized program and complete survey sample testing regularly in order to make sure the organization is producing test results that are accurate and compare favourably with other labs across the country. The cost of running quality control samples for all the various tests on the lab’s menu is also a cost that is helps the lab ensure the accuracy and precision of their test results. Having the infrastructure in place to support the QMS is a 3rd expense that must be born in order to make sure the testing site has consistency and reliability of testing. Dedicated resources need to be put in place, particularly staffing, to ensure that someone is responsible for things like reviewing and updating procedures, monitoring the Quality control and proficiency testing results, and participating in ongoing aspects of continuous improvement. Professional development and competency assessment for staff is also a costly undertaking but one that must be supported in order to ensure the quality of patient test results.
If these costs are not being met by the Laboratory, there will definitely be costs associated with a lack of quality performance. Costs associated with a lack of quality performance include the costs associated with failure to produce quality results. These include things like the cost of running repeat samples –reagents, consumables and time, the cost of paying staff to run the duplicate tests and perform problem shooting to determine where the issues lay, the costs of misdiagnosis and mistreatment of patients, and finally the costs of poor workmanship – loss of reputation and confidence from potential customers, including physicians and patients alike.
Overall, the benefits of performing high quality, accurate testing procedures far outweigh the costs associated with providing that service and the costs of poor quality significantly outweigh the costs of providing the high quality laboratory services.
Question
- What are some of the other costs associated with provision of quality laboratory services?
Review Questions
- Why is it important to have a Quality Management System in place in the Clinical Laboratory?
- What are the key elements of the QMS?
- List the 12 quality system essentials.
- What are the 3 components of the Path of Workflow? List 2 examples of processes that fit under each component.
- The does it mean to perform ethically in the clinical laboratory?
- The costs associated with poor quality work in the clinical lab, include which of the following?
- Staffing costs
- Reagent, calibrator, and control costs for repeat testing
- Professional development and continuing education costs
- Belonging to a proficiency testing program
- Hammerling, J.A. (2012). A review of medical errors in laboratory diagnostics and where we are today. Laboratory Medicine, 43(2), P.41-43. https://doi.org/10.1309 ↵
- Ibid, p.41 ↵
- A Quality Management System Model for Laboratory Services: Approved Guideline, 5th edition. p.1 ↵
- Canadian Standards Association., Noble, M., & Canadian Standards Association. (2010). The ISO 15189:2012 essentials: A practical handbook for implementing the ISO 15189:2012 standard for medical laboratories (p.2). Mississauga, Ont: Canadian Standards Association. ↵
- Ibid, p.8 ↵
- Canadian Standards Association., Noble, M., & Canadian Standards Association. (2010). The ISO 15189:2007 essentials: A practical handbook for implementing the ISO 15189:2007 standard for medical laboratories. Mississauga, Ont: Canadian Standards Association. ↵
