8. QSE Continual Improvement
Organizations are continually working on ways to improve services to their patients and to make the operations of the organization flow more smoothly. There never comes a time when no more improvements are needed – no organization is ever perfect! That brings us to the next Quality System Essential – continual improvement, commonly known as quality improvement or continuous quality improvement. Whatever name you use, the process remains the same. As with the other QSEs, there are policies, processes and procedures that are related to this QSE.[1]
Policies that apply to process improvement include policies to address the following activities
- Participation in quality improvement activities that deal with relevant areas and outcomes of patient care
- Systematic review of all laboratory processes to identify possible sources of errors and non-conformances that require corrective action
- Corrective action for identified process problems
- Evaluation of the effectiveness of such actions and improvements
Processes that relate to Process improvement include
- Establishing a mechanism for identifying opportunities for improvement
- Developing mechanisms to use to change processes that are found to need improvement
- Using defined strategies for process improvement
- Developing mechanisms to assess whether improvements that have been made are working effectively
Procedures needed to ensure that these processes are implemented effectively include documented instructions for
- Reviewing data collection from surveys, audits, etc.
- Applying the problem solving process
- Planning the proposed and suggested improvements
- Obtaining approval for implementations
- Measuring effectiveness of any changes
- Preparing reports to management about any changes and preventative actions taken
Process improvement takes place in all areas of the laboratory services and can affect patients in all areas of the hospital and beyond to the community. As we discussed previously, the pre-examination services provide the greatest area for improvement but all areas have services and processes that can be improved.
When a laboratory is looking at where they should focus improvement efforts, there are several sources that can support that work
- Feedback for employees
- Feedback from patients, physicians and other members of the healthcare team
- Feedback from external customers such as referral labs, private phlebotomy services, nursing homes, etc.
- Performance on external assessments from government and accreditation surveys
- Performance of quality indicators
- Performance on external quality audits
- Benchmarking information
- Analyses of non-conforming events, including trends
- Reports from other areas of the organization
- Literature reviews
There are also several questions that must be answered when considering any improvement process. They are[2]
- What needs to change?
- What should be selected for change now?
- What will change?
- How should the change happen?
- Did the change work as expected?
- Is the change continuing to work as expected?
According to the CLSI[3], it is very important for employees to recognize that they play both an important and ethical role in bringing forward areas of concern or areas where they recognize that improvement could be implemented to improve patient safety and the efficient, effective delivery of laboratory services. Teamwork plays a very important role in how successful any improvement efforts may be. Teams work through collaborative efforts and their efforts are very important in ensuring that the planned changes take place and are implemented as they were designed.
There are two ways of identifying improvements that can be achieved in laboratory services, or in the operation of any organization. These are improvements that arise from problem resolution and improvements that arise through preventive and corrective action.
Problem resolution is the result of taking remedial action – it is implemented to rectify a recognized error or nonconformance. For example, repeat testing done in response to losing a report would be one incidence where remedial action is the appropriate response.
Preventive and corrective action issues are often identified through quality indicators. For example, a laboratory may be collecting data on Turnaround Time to monitor how long it takes to get results back to the Emergency Dept. If the indicator review shows that the average time is gradually getting longer, a plan may be put in place to try to improve the TAT. This would be an example of corrective action that would be implemented to fix something that is not operating as required. Preventive action, on the other hand, is implemented when a potential problem has been identified that has not occurred yet. This action is implemented to prevent the possible problem from occurring down the road. Quality indicator data may show where trends or patterns are developing that are detrimental to the optimal level of service that should be in practice.
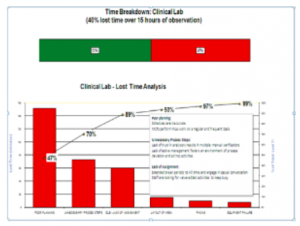
The Pareto Chart above shows the results of an audit in a laboratory where the management team was looking at “time lost” by employees over the course of a working day. As you can see, the chart was prepared in order of the number of times a factor occurred with the most commonly occurring factor at the front and the other factors following in descending order of occurrences. The Pareto Chart shows very clearly which factor was responsible for the bulk of the issues that happened during the audit time. Pareto analysis shows clearly that most of the time, 80% of the problems are caused by a few key causes (20%). With this information, now the management team can look at ways to reduce the lost time.
Another way quality improvement is practiced in laboratories is to enact preventative and corrective action through one of three different types of performance enhancements – LEAN, Six Sigma or Lean Six Sigma. These performance improvement methods are based on processes that improve time and reduce error. In this way, they are continually providing improved performance over time.
LEAN processes work by reducing non-value added steps or by finding ways to reduce the number of steps required to carry out a process. The purpose of LEAN is that it will reduce the length of time needed to perform testing processes overall. Several Labs in Nova Scotia have been working on implementing LEAN processes in their labs in recent years.
Six Sigma is a process that looks at ways to reduce error in the laboratory. The objective of this process is to reduce the errors by reducing the amount of variation in any process. It is based on a mathematical process where the reduction in errors can be monitored through mathematical calculations. According to Oks & Wescott, “as a breakthrough strategy, six sigma constitutes an evolving set of principles, fundamental practices, and tools”. [4]Using Six Sigma requires a strong leadership commitment by the organization and the empowerment of employees to work on continuously improving processes, products, and services. Although this process is not as commonly utilized in NS at this time, there are certainly incidences where it may be used.
Lean Six Sigma, as its name implies, is a combination of the principals of both LEAN and Six Sigma. By combining the LEAN focus on waste reduction with the Six Sigma focus of reducing waste by reducing variability and increasing efficiency, the overall capability of the entire process can be improved.
The four key concepts of this method include
- Delighting customers
- Improving processes
- Teamwork
- Data-driven decision making
There have been 8 common wastes identified in the use of LEAN and LEAN Six Sigma processes. They include:
- Rework required to remove defects
- Overproduction by supplying more than is needed to effectively operate
- Transporting the product without any value-added benefit
- Building a larger inventory than is needed for current use
- Waiting for an extended period between steps in the process
- Moving people or equipment more than is necessary to produce the final result
- Overwork where more is done than is required by the customer
- Any failure to use the talents of people appropriately.[5]
However, whichever process is used to help an organization identify opportunities for continual improvement, one vital component for success is effective Teamwork.
Once a problematic issue has been identified in laboratory service delivery, the problem must be properly identified before corrective action can be effectively implemented. This leads us to the problem resolution process. For example, when quality indicators identify that the TAT for getting results back to Emergency is taking too long, the lab has identified an issue. However, in order to fix the problem, the laboratory must find out where in the process the delays are actually occurring.
The first thing that must be done is to identify where in the process the problem that is causing results to be delayed occurs. Is it occurring in transport of specimens to the lab, in the time staff take to process the specimen once it arrives in the lab, or in the time the completed test sits on the analyzer before results are called to the Emergency department? It is counterproductive to try to fix the problem before it has been clearly identified. This process is called root cause analysis and must be utilized to ensure that the actions being considered to address a problem are actually getting to the original cause.
Determining the root cause of the problem is actually a part of the overall problem resolution process which includes several steps. In the first piece of the process the problem must be identified. It can be effective in looking at the pieces involved in a process and then which piece of the process is actually causing the problem. One very effective tool that can be used in the root cause analysis is the Cause and Effect diagram. Looking at each component of the situation and breaking out the implications of changes to each part of the process is very valuable in finding the root cause. There are several other tools that are also valuable for continual improvement initiatives and they include:
- Brainstorming
- Failure modes and critical effects analysis (FEMA)
- 5S/workplace organization
- Flow chart / Process map
- Gap analysis
- Histogram
- Pareto Chart
- Scatter plot
- Cause and Effect diagram
Cause and Effect Diagram
Problem Resolution Process
- Problem identification (problem statement)
- Analysis of current processes and collection of data
- Determination of the problem’s root cause
- Generation of ideas for possible solution and determining the possible advantages and disadvantages of each option
- Selection and implementation of solution
- Monitoring and feedback of the improved process
Once the cause of the problem has been identified, then a plan can be developed and implemented to correct this problem. For example, if the transportation of specimens from the Emergency Department to the Laboratory is taking an unreasonable length of time, then the options available for fixing this problem must be investigated. Would it be possible to install a tube system? Should more porter staff be hired to deliver these specimens? Is a new process needed to notify porters when the specimen has been collected?
After all of the possible options have been identified, then it is necessary to look at the pros and cons of implementing each option. What is the cost of installing a tube system compared to hiring more porter staff? Is there space to install a tube system? Would the porters be kept busy throughout the shift or would that not be a good use of resources? There are several factors that must be taken into consideration before the decision is made to implement any one solution. Once that decision has been made, then it must be implemented, monitored and followed up to see it is in fact improving the process. In this example, is the TAT from specimen collection to result reporting being shortened and therefore improving the speed with which patients can receive diagnosis and treatment in the Emergency Department?
Exercise
Use the space below to draw in a Cause and Effect Diagram to Brainstorm the possible causes of a problem in taking too long to get blood specimens from Outpatient collections to the lab receiving area.
The most commonly used tool to implement a process improvement solution once it is determined, is the Plan-Do-Check-Act process. This model of implementing improvements has been around for many years and is used across industries. It is not exclusive to healthcare quality but to a wide variety of industries because it has proven to be an effective process.
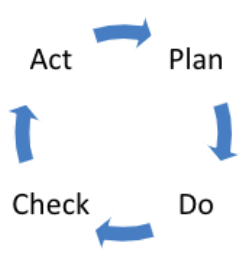
Plan: Develop a mission-consistent, customer-focused action plan.
- Study the situation
- Decide what needs to be done to improve it and develop options to make improvements
- Decide on which option is the most likely to improve the process
- Develop a plan and a measurement process to monitor the improved process
Do: Put the plan into action
- Implement the plan as a pilot
- Collect performance data
Check: Has the change created the intended improvement?
- Determine whether the plan worked effectively
- Study the results of the data collected
- Identify any unexpected benefits or unanticipated problems
Act: Decide what to do next
- If the plan worked, implement the plan on a regular, ongoing basis and continue to monitor and make adjustments as needed
- If the plan didn’t result in an improved process, try something else. Go back to the planning step and start over.
Continual improvement is not a static process. It continues to develop and improve over time as new methods and supports become available, as new problems are identified and technology evolves over time.
Process Improvement Exercise
The Laboratory has been receiving complaints over the past several months that reports going to the Renal Clinic are taking too long to reach the requesting physicians. This issue is delaying the treatment phase for the patients and in some cases is leading to permanent deteriorating kidney function for the patient. How should the lab address this issue?
What steps need to be taken first? How will they be carried out?
What are some of the tools that may be used to analyze the data?
Once the root cause of the problem has been identified, what is the next step?
Describe the PDCA process that will be implemented to resolve the issue.
Quality Improvement Exercise
PDCA Process
Issue: The Lab is looking at implementing a new procedure in Clinical Chemistry
Plan: (Develop an action plan – How, when, where)
Do: (put the plan into action)
Check: (Monitor ongoing work)
Act: (What do we do from here?)
- If it is working ……
- If it is not working …..
Review Questions
- What are the 2 ways of identifying where improvements are needed in the clinical laboratory? Please explain how each type works.
- How does LEAN work?
- What is the purpose of Six Sigma?
- List the steps in the problem resolution process.
- What is root cause analysis and what is it used for?
- What is a Cause and Effect diagram used for?
- Berte, L. (2019). A quality management system model for laboratory services [GP26-A4] (5th ed.). Wayne, PA: Clinical and Laboratory Standards Institute. p.113 ↵
- GP22-A3, p.3 ↵
- Ibid. p.4 ↵
- Okes, D., & Westcott, T. (2001). The certified quality manager handbook (2nd ed.). Milwaukee, Wisconsin: American Society for Quality Press. P.164. ↵
- Berte, L. (2019). A quality management system model for laboratory services [GP26-A4] (5th ed.). Wayne, PA: Clinical and Laboratory Standards Institute. ↵
