6. QSE Process Management
Process Management refers to all aspects of the Quality Management System. There should be written policies, followed by process maps and written procedures for all of the components of the QMS. Listed below are the components of Process Management that are identified by the CLSI in the document A Quality Management System Model for Laboratory Services: Approved Guideline, 5th edition (GP26-A4, p.75).
Policies included in Process Management are
- Analysis and design of all work processes, including the laboratory’s Path of Work Flow and QSE activities
- Compliance with government, accreditation, and industry standards
- Process validation and/or verification
- Process control
- Change Management
Processes that are part of this QSE include
- Processes for the analysis and design of all new and existing processes
- Processes for the validation and verification of all processes
- Processes for the development, implementation, and ongoing monitoring of quality control plans
- Processes to manage change within the laboratory
Procedures, which are tracked through documents and records, are also part of this QSE. They include
- Designing and documenting work processes
- Performing validations and/or verifications for specific equipment and procedures
- Performing and monitoring quality control plans, and applying qc rules to both detect errors and avoid false rejection
- Defining acceptance and rejection criteria
- Performing statistical analysis
- Identifying and assessing the need for change
- Evaluating the effects of change on the other services of the laboratory
- Monitoring, recording and communicating changes
All of the policies, processes and procedures are important to ensure that the laboratory is performing tests to meet established expectations. This may be something as simple as writing a policy to say that the laboratory management controls all changes that take place within the laboratory. A process map in the form of a flowchart may lay out the way this policy is implemented with regard to equipment validation. A corresponding procedure that might fit under this process would be the step by step procedure used to perform validation on a new chemistry analyzer. Whatever the process that requires management, there must be established documentation to guide the fulfilment of this process. In this module, we are going to focus on several important areas of process management within Laboratory Services.
Analyzing, Designing, and Documenting all the processes of pre-examination, examination, and post-examination, as well as all aspects of the QSEs, is achieved through several steps:
- Documentation of processes
- Development of process maps or flowcharts
- Development and implementation of well-written procedures
- The creation of records to track what has been done
Documents and Records will be discussed more fully in the QSE by that name. For the purposes of this module it is important to note that, in order for the QMS to meet the expectations of customers and external agencies, a thorough system of documentation must be established and followed. Accreditation of Laboratory Services and other regulatory bodies require it.
Can you list who some of these bodies might be?
Flowchart
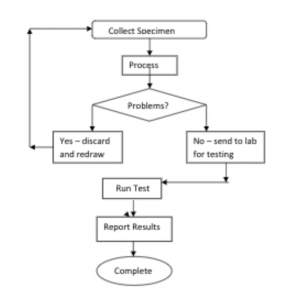
The following flowchart shows a simplified process to be followed for collecting, preparing and testing a patient specimen. As you can see, several procedures flow out of this process – phlebotomy collection procedure, specimen processing procedure, transport of specimen procedure, testing procedure and finally, reporting the test result.
Process Validation is used for validating testing methods, as well as for validating analyzers when they are purchased, to ensure that they are running with the correct degree of accuracy and precision for the testing methods you want to perform. In the clinical laboratory today, most method validations are performed on commercially developed test methods. Validation is the confirmation, obtained through objective evidence, that all the requirements of an analyzer or testing methods have been met.
- Accuracy studies to determine the trueness of measurement
- Precision studies to determine the ability to reproduce the same result through repeated analysis
- Analytical sensitivity studies that check the ability to detect the presence of the desired analyte
- Analytical specificity studies to confirm that the desired analyte is the only one being measured
- Comparison studies to compare results from the old analyzer to the new one, or to compare results of a new methodology with a reference method
- Studies that impact the uncertainties that may affect the accuracy of measurement such as sample collection and sample preparation processes, selection of calibrators and reagents, and the operator’s skill and experience.
As a general rule, validations are performed on analyzers and kit methods by the manufacturer before the products are placed on the market. If a method is developed in-house by laboratory staff, the validation studies would have to be performed by the laboratory before the procedure was put into active use for reporting patient results.
Because they have already had vigorous validation tests performed by the manufacturer, in most cases the role of the lab is to ensure that the test is performing as it was described by the manufacturer and to ensure that it works in the specific clinical laboratory where testing will occur under normal working conditions. This is known as Process Verification.
There are several factors that could affect performance in a given laboratory. The testing laboratory will want to check that the accuracy and precision, and the sensitivity and specificity of the testing process do perform as expected. They will also want to determine an acceptable reference range for the analytes being tested for that laboratory’s particular patient population. Various environmental factors may affect the ranges from one geographical location to another.
Many processes in laboratories are not direct testing processes. Pre-examination and post-examination processes must also be validated or verified before they are put into regular usage. These processes use different means of validating or verifying that they are working effectively. For example, if the laboratory changed the means of reporting lab results so that results were transmitted electronically to the ordering physician, what measures could the laboratory use to verify that this process was working effectively?
Once verifications have been completed and approved, the laboratory must ensure that all documentation is in order and that all methodologies are approved. Education and training needs for staff must also be in place and documented before the actual changes can be implemented on an ongoing, permanent basis. From that point onward, whenever a change occurs within the system that could have an impact on patient results or the reliability of the method, re-verification procedures must be completed. Some examples of changes that could impact testing might include a change to the collection tube being used to collect the patient specimen or a change to the transportation process for specimens being moved between the collection site and the actual testing site.
List some of the factors in the transportation process that could have an impact on test results.
Process Control forms the foundation of accurate, precise patient test resulting. It is needed to ensure maximum effectiveness and efficiency in all aspects of the laboratory’s Path of Workflow.
There are several processes and tools that fall under the category of Process Control. Although the most common of these are quality controls and statistical techniques, proficiency testing programs, non-conformance events and quality indicators are also considered to be process controls that are commonly used in clinical laboratories. Since we will be talking about each of these processes in other QSEs, we will leave a thorough discussion of their uses until later.
To ensure accurate, precise test results, all methodologies for testing in the clinical laboratory must have quality controls attached to them. For example, in the Transfusion area, the refrigerators that store blood product are monitored for temperature to ensure that the products do not get too warm or too cold. This control process ensures that the product is still good to use for transfusing the patient.
All testing procedures themselves require controls to be run on a regular basis to ensure that the testing process is performing according to specifications. If the quality control results are outside the expected range for testing a particular analyte, the patient results for that analyte cannot be reported and an investigation must occur to determine the cause of the out-of-control result. Otherwise, inaccurate patients test results could lead to inaccurate diagnosis and treatment of the patients.
Similarly, all batches of media that are produced for growing organisms in Microbiology must have quality control procedures performed on them to make sure that specific organisms will grow on those plates, to ensure that organisms that should be inhibited do not grow, and also to make sure the plates are not contaminated by some unexpected bacteria. Otherwise, the technologist cannot be confident that the results produced on that plate after incubation are acceptable.
Exercise
What are some other examples of QC Usage?
Gram Stain
Refrigerators
Hematology Controls
Chemistry Controls
