14 Calorimetry for Determination of Specific Heat Capacity
Calorimetry for Determination of Specific Heat Capacity
This lab is designed to align with AAOT science outcome #1: Gather, comprehend, and communicate scientific and technical information in order to explore ideas, models, and solutions and generate further questions.
Materials
- digital device with spreadsheet program
- digital device with internet access
ObjectiveS
- Model the contents of a calorimeter as an isolated system to determine the unknown specific heat value of an unidentified metal.
- Identify the metal by comparing the determined specific heat value to tabulated values.
- Apply an improved model that includes calorimeter components in the isolated system and compare the results with the model that excludes the calorimeter from the system.
- Estimate the uncertainty in the specific heat value caused by cooling of the hot metal during the transfer to the calorimeter.
Methods
Experimental Methods
The video below shows the methods used to warm a piece of metal in a hot water bath and then transfer the metal to a Styrofoam cup filled with water inside a calorimeter. The Styrofoam cup provides insulation, and the cup itself is suspended within an aluminum cylinder with a layer of air between to provide additional insulation. The shiny metal cylinder also reduces radiative exchange of thermal energy. The cylinder itself is then suspended within a larger metal shell, once again separated by a layer of insulating air. A lid prevents significant convection or evaporation, but does have a hole to allow insertion of of a thermometer to monitor the contents of the calorimeter. Due to the hold in the lid, the calorimeter is an open system. If we assume the hole is sufficiently small that there is negligible exchange of particles in/out of the calorimeter then we can treat the contents as a ___________ system. If we also assume that the insulation is essentially perfect and the heat transfer in/out of the calorimeter is negligible then we can treat the contents as an _____________ system.
Analysis Methods
1) Modeling the metal and the water inside the calorimeter as an isolated system allows us to set the net thermal energy transfer (heat) of the system to zero. Therefore, the heat gained by the water must equal the heat lost by the metal. Using this model, symbolically derive an equation for the specific heat of the metal ($c_m$). Your result should depend on the following variables that are seen in the video: mass of metal ($m_m$), initial metal temperature ($T_m$), specific heat of water ($c_w$), mass of water ($m_w$), initial water temperature ($T_w$), and final temperature of the combination ($T_f$). Show all of your work:
2) Enter the values from the video into your model for the metal specific heat and calculate a value. Show you work.
Conclusions
3) Look at this table of specific heat values and compare your value to those listed to identify the metal in this experiment. List the value of the metal you chose from the list and calculate a % difference from the value found in this experiment.
Further Questions
4) List some possible sources of uncertainty in our determination of the specific heat value that were related to the experimental methods and measurement tools, but not caused by mistakes in calculations.
5) Our model neglected any temperature change by the Styrofam cup in the calorimeter. Now include the Styrofoam cup in the model and recalculate the specific heat value of the metal. Assume the initial and final temperatures of the Styrofoam were the same as the water it contained. Look up a value for Styrofoam specific heat capacity from a reliable source and provide a full URL for your source. Show all work.
6) How much did the determined specific heat value change when accounting for heating of the Styrofoam cup, and did it increase or decrease? Calculate a % difference between the two values produced by the two models.
7) Did accounting for this effect bring the specific heat value closer to a different metal the the one you identified previously? Explain. Does it appear to be necessary to include the Styrofoam cup in the analysis for this type of experiment? Explain your reasoning.
Our analysis methods neglected any cooling of the metal during the transfer from the hot water bath to the calorimeter. The following infrared images indicate that the metal is radiating and that air surrounding the metal is undergoing natural convection (which implies that the air is first warmed by conduction from the metal).
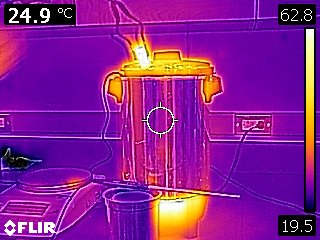
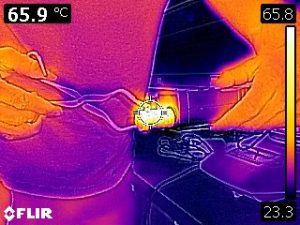
To estimate how much cooling may have occurred, another experiment was performed. The metal was once again removed from the hot water bath and it’s temperature was measured and recorded by an infrared thermometer as seen in the following video.
8) How much did the temperature of the metal change during three seconds in the air just after being removed from the hot water bath?
9) Assume the same temperature change occurred for the metal in the original calorimetry experiment and that the metal was actually that much colder than you originally thought. Recalculate the metal specific heat with the expected lower initial temperature value. Show all work.
10) How much did the determined specific heat value change when accounting for cooling of the metal during transfer, and did it increase or decrease? Calculate a % difference between the two values produced by the two models.
11) Did accounting for this effect bring the specific heat value closer to a different metal than the one you identified previously? Explain. Does it appear necessary to be concerned about the heat loss during transfer to the calorimeter for this type of experiment? Explain your reasoning. (Consider the error in determining specific heat that you discovered was caused by these effects and compared to the difference in specific heat between metals).
12) The metal used in the experiment was actually zink. Was that the metal you identified?
13) Calculate a % difference between the final experimental specific heat capacity of the metal and the tabulated value for zink (look it up and provide a citation for your source).

