10.7 Valence Bond Theory and Hybrid Orbitals
Learning Objectives
- Gain an understanding of valence bond theory.
- Gain an understanding of hybrid orbitals.
Valence Bond Theory
Earlier we saw that covalent bonding requires the sharing of electrons between two atoms, so that each atom can complete its valence shell. But how does this sharing process occur? Remember that we can only estimate the likelihood of finding an electron in a certain area as a probability. This probability is represented as a distribution in space that we call an atomic orbital (Figure 10.5 “Representations of s and p atomic orbitals”)
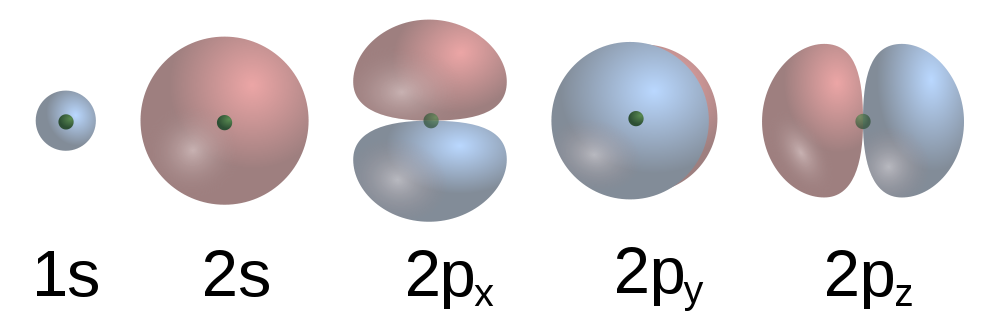
The valence bond theory states that atoms in a covalent bond share electron density through the overlapping of their valence atomic orbitals. This creates an area of electron pair density between the two atoms. Since these electrons are simultaneously attracted to both nuclei, the electron pair holds the two atoms together.
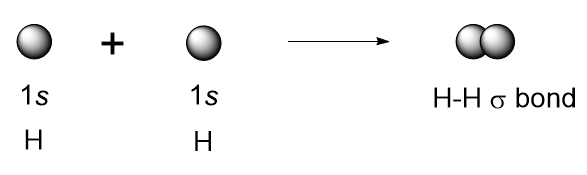
Let’s examine the simplest case of atomic overlap resulting in a covalent bond, the formation of H2 from two hydrogen atoms (Figure 10.6 “A diagram showing the overlap of s orbitals of two hydrogen atoms to form H2“). The 1s orbitals of the two hydrogens approach each other and overlap to form a bond that has cylindrical symmetry known as a sigma bond (σ bond). Repulsion forces between the two nuclei and between the two electrons are also present. The optimal distance between atoms, which maximizes the attractive forces and minimizes the repulsive forces, gives the H-H sigma bond a length of 74 pm.
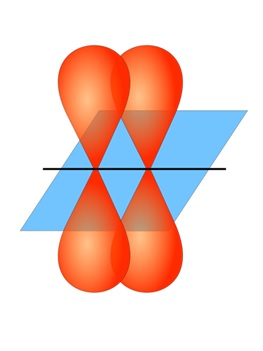
For molecules that contain double or triple bonds, one of these bonds is a sigma bond, and the remaining multiple bonds are a different type of bond known as a pi bond (π bond). Pi bonds result from the sideways overlap of p orbitals, placing electron density on opposite sides of the internuclear axis (Figure 10.7 “Pi bond diagram showing sideways overlapping of p orbitals”).
Hybrid Orbitals
sp3 hybridization
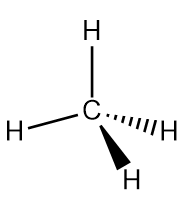
A problem arises when we apply the valence bond theory method of orbital overlap to even simple molecules like methane (CH4) (Figure 10.8 “Methane”). Carbon (1s2 2s2 2p2) only has two unpaired valence electrons that are available to be shared through orbital overlap, yet CH4 has four C-H σ bonds!
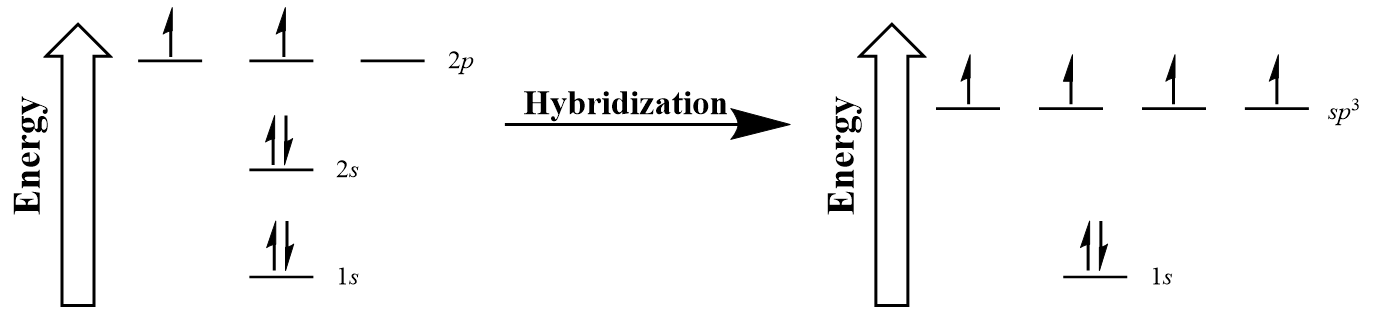
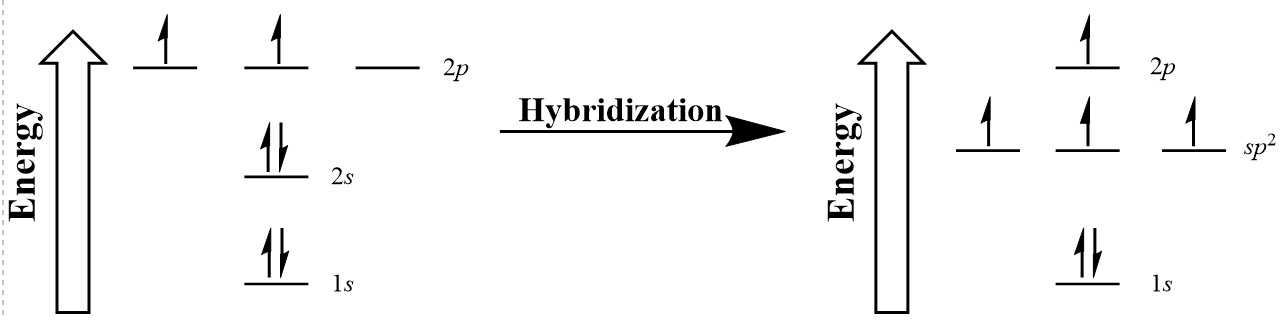
In 1931, Linus Pauling (Figure 10.9) proposed a mathematical mixing of atomic orbitals known as hybridization. The 2s and three 2p orbitals are averaged mathematically through hybridization to produce four degenerate sp3 hybrid orbitals (Figure 10.10 “Hybridization of carbon to generate sp3 orbitals”). Note that in hybridization, the number of atomic orbitals hybridized is equal to the number of hybrid orbitals generated.
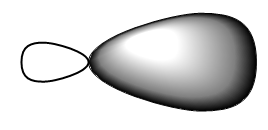
The sp3 orbitals, being a combination of a spherical s orbital and propeller- (or peanut-) shaped p orbital, give an unsymmetrical propeller shape where one lobe of the orbital is larger (fatter) than the other (Figure 10.11 “An sp3 hybridized atomic orbital”). This larger lobe is typically used for orbital overlap in covalent bonding.
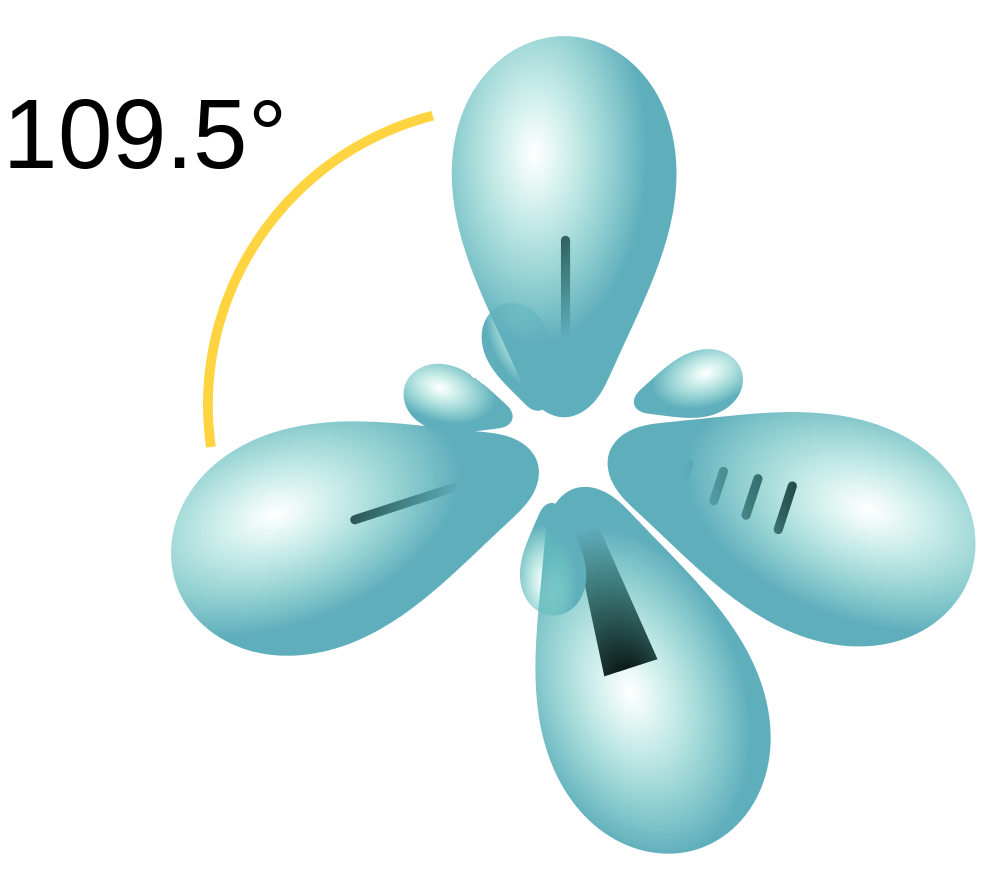
According to VSEPR theory, the four degenerate orbitals will arrange as far apart from each other as possible, giving a tetrahedral geometry with each orbital 109.5o apart (Figure 10.12 “A carbon atom’s four tetrahedral sp3 hybridized orbitals”).
sp2 hybridization
Let’s examine another simple molecule, ethene (C2H4) (Figure 10.13 “Ethene”). Each carbon of ethene is bonded to two hydrogens and a carbon. There is also a double bond between the two carbons.
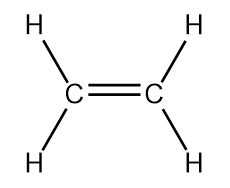
Both the unhybridized atomic orbitals of carbon and the sp3 hybridization we just examined do not explain the bonding observed in ethene. In this case, only the 2s and two of the 2p orbitals hybridize to give three new sp2 hybridized orbitals capable of forming the three σ bonds of each carbon in ethene (Figure 10.14 “Hybridization of carbon to generate sp2 orbitals”).
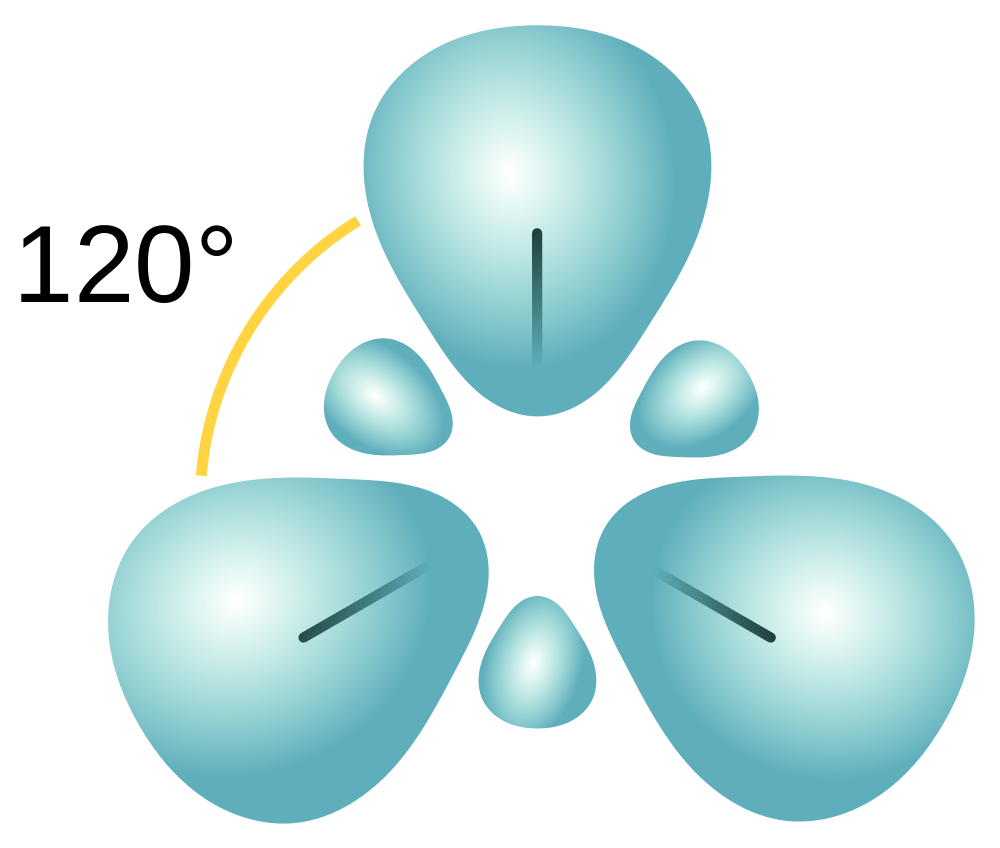
The three hybridized orbitals arrange in a trigonal planar structure with a bond angle of 120o following VSEPR (Figure 10.15 “A carbon atom’s trigonal planar sp2 hybridized orbitals”).
The unhybridized 2p orbital in both carbons are left available to form the double bond’s π bond.
sp hybridization
The final example of hybridization we will examine is the molecule ethyne (C2H2) (Figure 10.16 “Ethyne”).
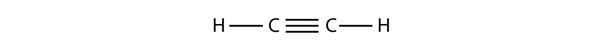
The carbons in ethyne are each sigma bonded to a single hydrogen, but triple bonded to each other. Again, the models of hybridization we have looked at so far are insufficient to explain the bonding pattern observed. In ethyne, only the 2s and one of the 2p orbitals hybridize to give two new sp hybridized orbitals capable of forming the two σ bonds of each carbon in ethyne (Figure 10.17 “Hybridization of carbon to generate sp orbitals”).
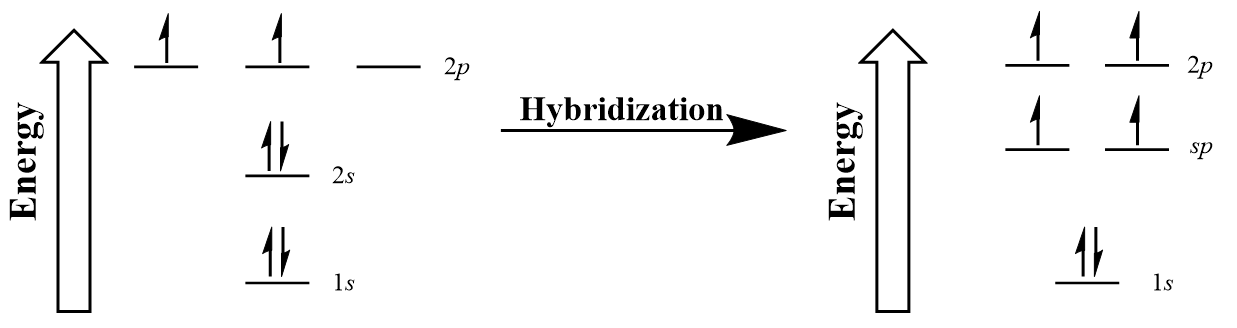
The two hybridized sp orbitals arrange linearly with a bond angle of 180o following VSEPR (Figure 10.18 “A carbon atom’s linear sp hybridized orbitals”).
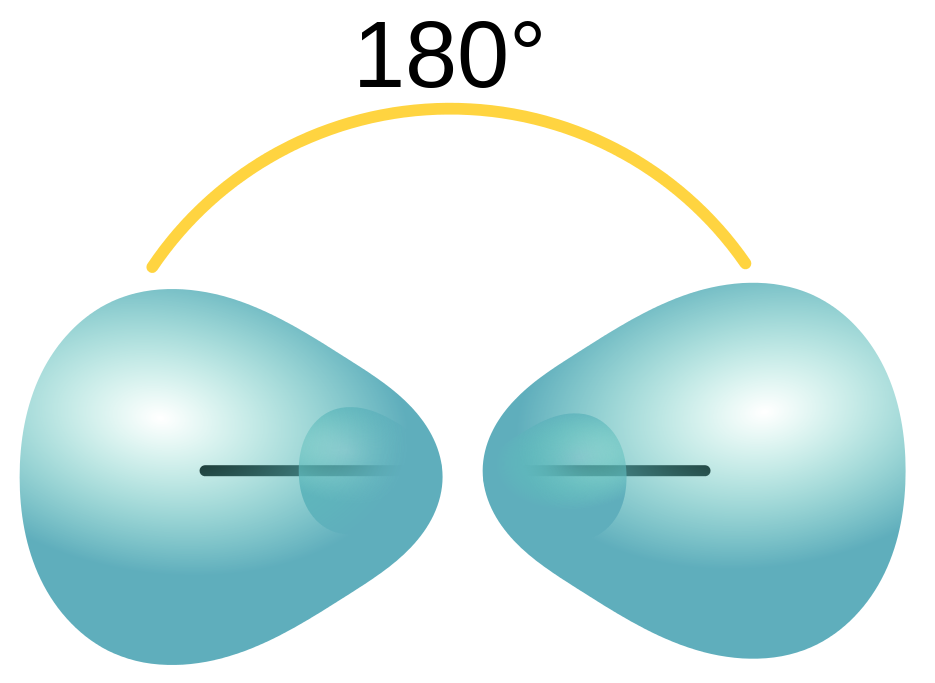
The two remaining unhybridized 2p orbitals in both carbons are left available to form the triple bond’s two π bonds.
Other hybridizations
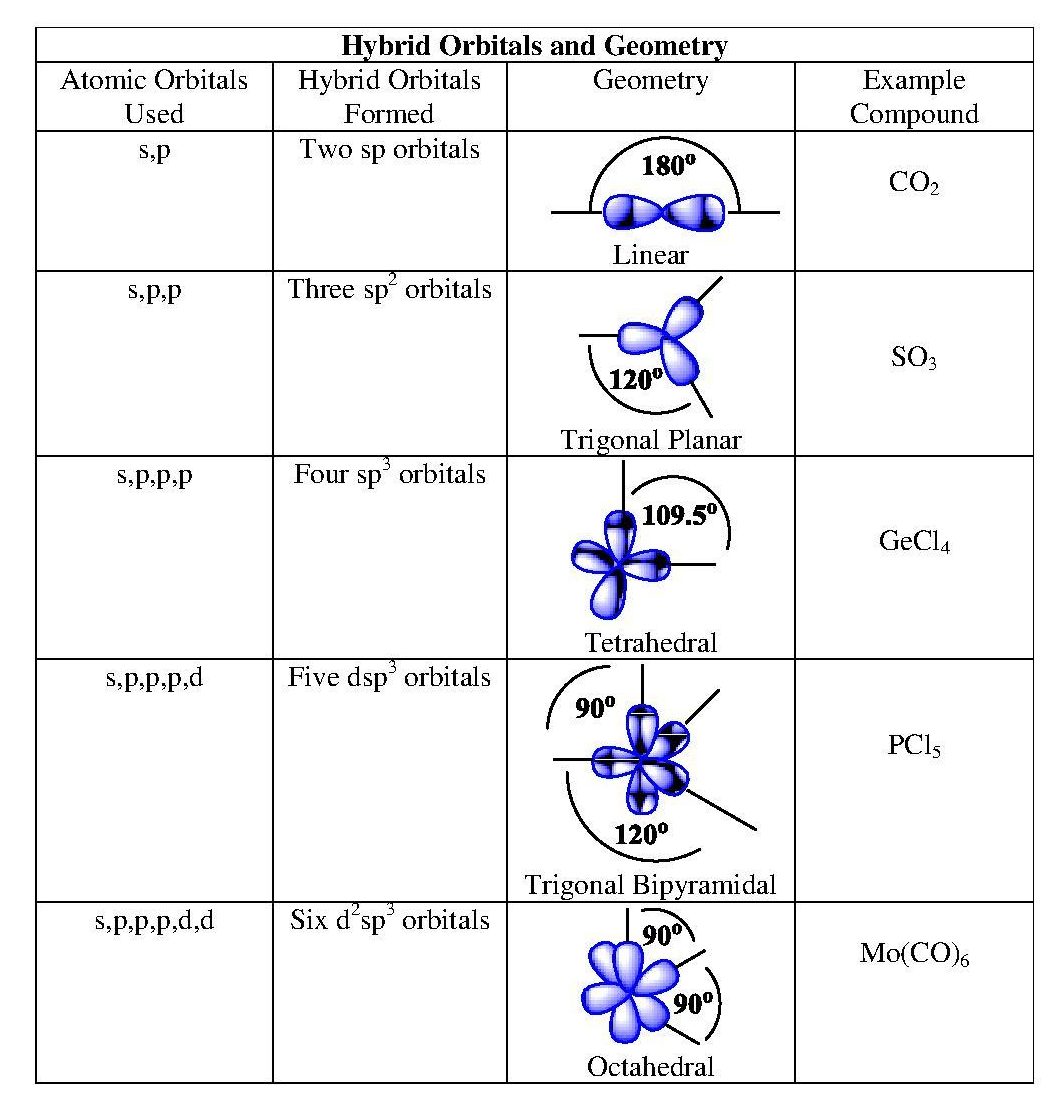
Other hybridizations are possible, allowing us to apply valence bond theory to explain the bonding patterns observed in most real molecules. Some additional hybridizations are summarized in Table 10.5 “Hybrid Orbitals and Geometry.”
Key Takeaways
- Valence bond theory explains bonding through the overlap of atomic orbitals.
- Atomic orbitals can be hybridized mathematically to better explain actual bonding patterns.

