125 Introduction
[latexpage]
Learning Objectives
- Nuclear Structure and Stability
- Nuclear Equations
- Radioactive Decay
- Transmutation and Nuclear Energy
- Uses of Radioisotopes
- Biological Effects of Radiation
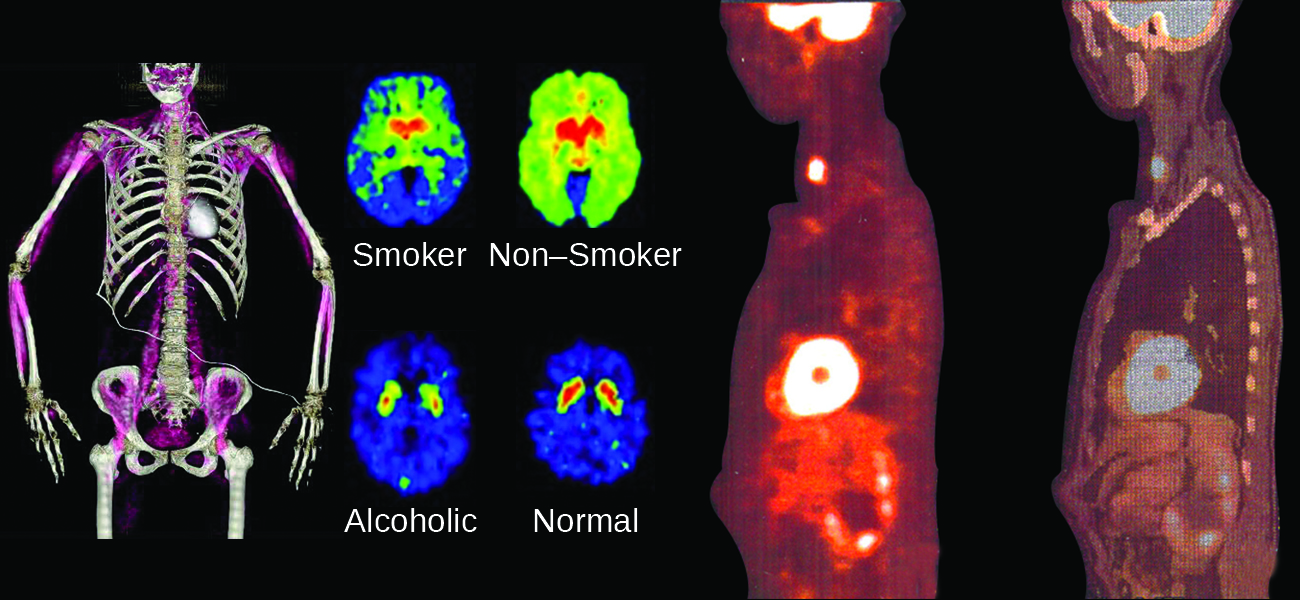
The chemical reactions that we have considered in previous chapters involve changes in the electronic structure of the species involved, that is, the arrangement of the electrons around atoms, ions, or molecules. Nuclear structure, the numbers of protons and neutrons within the nuclei of the atoms involved, remains unchanged during chemical reactions.
This chapter will introduce the topic of nuclear chemistry, which began with the discovery of radioactivity in 1896 by French physicist Antoine Becquerel and has become increasingly important during the twentieth and twenty-first centuries, providing the basis for various technologies related to energy, medicine, geology, and many other areas.

