129 Transmutation and Nuclear Energy
[latexpage]
Learning Objectives
By the end of this section, you will be able to:
- Describe the synthesis of transuranium nuclides
- Explain nuclear fission and fusion processes
- Relate the concepts of critical mass and nuclear chain reactions
- Summarize basic requirements for nuclear fission and fusion reactors
After the discovery of radioactivity, the field of nuclear chemistry was created and developed rapidly during the early twentieth century. A slew of new discoveries in the 1930s and 1940s, along with World War II, combined to usher in the Nuclear Age in the mid-twentieth century. Scientists learned how to create new substances, and certain isotopes of certain elements were found to possess the capacity to produce unprecedented amounts of energy, with the potential to cause tremendous damage during war, as well as produce enormous amounts of power for society’s needs during peace.
Synthesis of Nuclides
Nuclear transmutation is the conversion of one nuclide into another. It can occur by the radioactive decay of a nucleus, or the reaction of a nucleus with another particle. The first manmade nucleus was produced in Ernest Rutherford’s laboratory in 1919 by a transmutation reaction, the bombardment of one type of nuclei with other nuclei or with neutrons. Rutherford bombarded nitrogen atoms with high-speed α particles from a natural radioactive isotope of radium and observed protons resulting from the reaction:
The \({}_{\phantom{\rule{0.5em}{0ex}}8}^{17}\text{O}\) and \({}_{1}^{1}\text{H}\) nuclei that are produced are stable, so no further (nuclear) changes occur.
To reach the kinetic energies necessary to produce transmutation reactions, devices called particle accelerators are used. These devices use magnetic and electric fields to increase the speeds of nuclear particles. In all accelerators, the particles move in a vacuum to avoid collisions with gas molecules. When neutrons are required for transmutation reactions, they are usually obtained from radioactive decay reactions or from various nuclear reactions occurring in nuclear reactors. The Chemistry in Everyday Life feature that follows discusses a famous particle accelerator that made worldwide news.
Located near Geneva, the CERN (“Conseil Européen pour la Recherche Nucléaire,” or European Council for Nuclear Research) Laboratory is the world’s premier center for the investigations of the fundamental particles that make up matter. It contains the 27-kilometer (17 mile) long, circular Large Hadron Collider (LHC), the largest particle accelerator in the world ((Figure)). In the LHC, particles are boosted to high energies and are then made to collide with each other or with stationary targets at nearly the speed of light. Superconducting electromagnets are used to produce a strong magnetic field that guides the particles around the ring. Specialized, purpose-built detectors observe and record the results of these collisions, which are then analyzed by CERN scientists using powerful computers.

In 2012, CERN announced that experiments at the LHC showed the first observations of the Higgs boson, an elementary particle that helps explain the origin of mass in fundamental particles. This long-anticipated discovery made worldwide news and resulted in the awarding of the 2013 Nobel Prize in Physics to François Englert and Peter Higgs, who had predicted the existence of this particle almost 50 years previously.
Famous physicist Brian Cox talks about his work on the Large Hadron Collider at CERN, providing an entertaining and engaging tour of this massive project and the physics behind it.
View a short video from CERN, describing the basics of how its particle accelerators work.
Prior to 1940, the heaviest-known element was uranium, whose atomic number is 92. Now, many artificial elements have been synthesized and isolated, including several on such a large scale that they have had a profound effect on society. One of these—element 93, neptunium (Np)—was first made in 1940 by McMillan and Abelson by bombarding uranium-238 with neutrons. The reaction creates unstable uranium-239, with a half-life of 23.5 minutes, which then decays into neptunium-239. Neptunium-239 is also radioactive, with a half-life of 2.36 days, and it decays into plutonium-239. The nuclear reactions are:
Plutonium is now mostly formed in nuclear reactors as a byproduct during the decay of uranium. Some of the neutrons that are released during U-235 decay combine with U-238 nuclei to form uranium-239; this undergoes β decay to form neptunium-239, which in turn undergoes β decay to form plutonium-239 as illustrated in the preceding three equations. It is possible to summarize these equations as:
Heavier isotopes of plutonium—Pu-240, Pu-241, and Pu-242—are also produced when lighter plutonium nuclei capture neutrons. Some of this highly radioactive plutonium is used to produce military weapons, and the rest presents a serious storage problem because they have half-lives from thousands to hundreds of thousands of years.
Although they have not been prepared in the same quantity as plutonium, many other synthetic nuclei have been produced. Nuclear medicine has developed from the ability to convert atoms of one type into other types of atoms. Radioactive isotopes of several dozen elements are currently used for medical applications. The radiation produced by their decay is used to image or treat various organs or portions of the body, among other uses.
The elements beyond element 92 (uranium) are called transuranium elements. As of this writing, 22 transuranium elements have been produced and officially recognized by IUPAC; several other elements have formation claims that are waiting for approval. Some of these elements are shown in (Figure).
| Preparation of Some of the Transuranium Elements | |||
|---|---|---|---|
| Name | Symbol | Atomic Number | Reaction |
| americium | Am | 95 | \({}_{\phantom{\rule{0.5em}{0ex}}94}^{239}\text{Pu}+{}_{0}^{1}\text{n}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{\phantom{\rule{0.5em}{0ex}}95}^{240}\text{Am}+{}_{-1}^{\phantom{\rule{0.5em}{0ex}}0}\text{e}\) |
| curium | Cm | 96 | \({}_{\phantom{\rule{0.5em}{0ex}}94}^{239}\text{Pu}+{}_{2}^{4}\text{He}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{\phantom{\rule{0.5em}{0ex}}96}^{242}\text{Cm}+{}_{0}^{1}\text{n}\) |
| californium | Cf | 98 | \({}_{\phantom{\rule{0.5em}{0ex}}96}^{242}\text{Cm}+{}_{2}^{4}\text{He}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{\phantom{\rule{0.5em}{0ex}}98}^{245}\text{Cf}+{}_{0}^{1}\text{n}\) |
| einsteinium | Es | 99 | \({}_{\phantom{\rule{0.5em}{0ex}}92}^{238}\text{U}+15{}_{0}^{1}\text{n}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{\phantom{\rule{0.5em}{0ex}}99}^{253}\text{Es}+7{}_{-1}^{\phantom{\rule{0.5em}{0ex}}0}\text{e}\) |
| mendelevium | Md | 101 | \({}_{\phantom{\rule{0.5em}{0ex}}99}^{253}\text{Es}+{}_{2}^{4}\text{He}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{101}^{256}\text{Md}+{}_{0}^{1}\text{n}\) |
| nobelium | No | 102 | \({}_{\phantom{\rule{0.5em}{0ex}}96}^{246}\text{Cm}+{}_{\phantom{\rule{0.5em}{0ex}}6}^{12}\text{C}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{102}^{254}\text{No}+4{}_{0}^{1}\text{n}\) |
| rutherfordium | Rf | 104 | \({}_{\phantom{\rule{0.5em}{0ex}}98}^{249}\text{Cf}+{}_{\phantom{\rule{0.5em}{0ex}}6}^{12}\text{C}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{104}^{257}\text{Rf}+4{}_{0}^{1}\text{n}\) |
| seaborgium | Sg | 106 | \(\begin{array}{l}{}_{\phantom{\rule{0.5em}{0ex}}82}^{206}\text{Pb}+{}_{24}^{54}\text{Cr}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{106}^{257}\text{Sg}+3{}_{0}^{1}\text{n}\\ {}_{\phantom{\rule{0.5em}{0ex}}98}^{249}\text{Cf}+{}_{\phantom{\rule{0.5em}{0ex}}8}^{18}\text{O}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{106}^{263}\text{Sg}+4{}_{0}^{1}\text{n}\end{array}\) |
| meitnerium | Mt | 107 | \({}_{\phantom{\rule{0.5em}{0ex}}83}^{209}\text{Bi}+{}_{26}^{58}\text{Fe}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{109}^{266}\text{Mt}+{}_{0}^{1}\text{n}\) |
Nuclear Fission
Many heavier elements with smaller binding energies per nucleon can decompose into more stable elements that have intermediate mass numbers and larger binding energies per nucleon—that is, mass numbers and binding energies per nucleon that are closer to the “peak” of the binding energy graph near 56 (see (Figure)). Sometimes neutrons are also produced. This decomposition is called fission, the breaking of a large nucleus into smaller pieces. The breaking is rather random with the formation of a large number of different products. Fission usually does not occur naturally, but is induced by bombardment with neutrons. The first reported nuclear fission occurred in 1939 when three German scientists, Lise Meitner, Otto Hahn, and Fritz Strassman, bombarded uranium-235 atoms with slow-moving neutrons that split the U-238 nuclei into smaller fragments that consisted of several neutrons and elements near the middle of the periodic table. Since then, fission has been observed in many other isotopes, including most actinide isotopes that have an odd number of neutrons. A typical nuclear fission reaction is shown in (Figure).
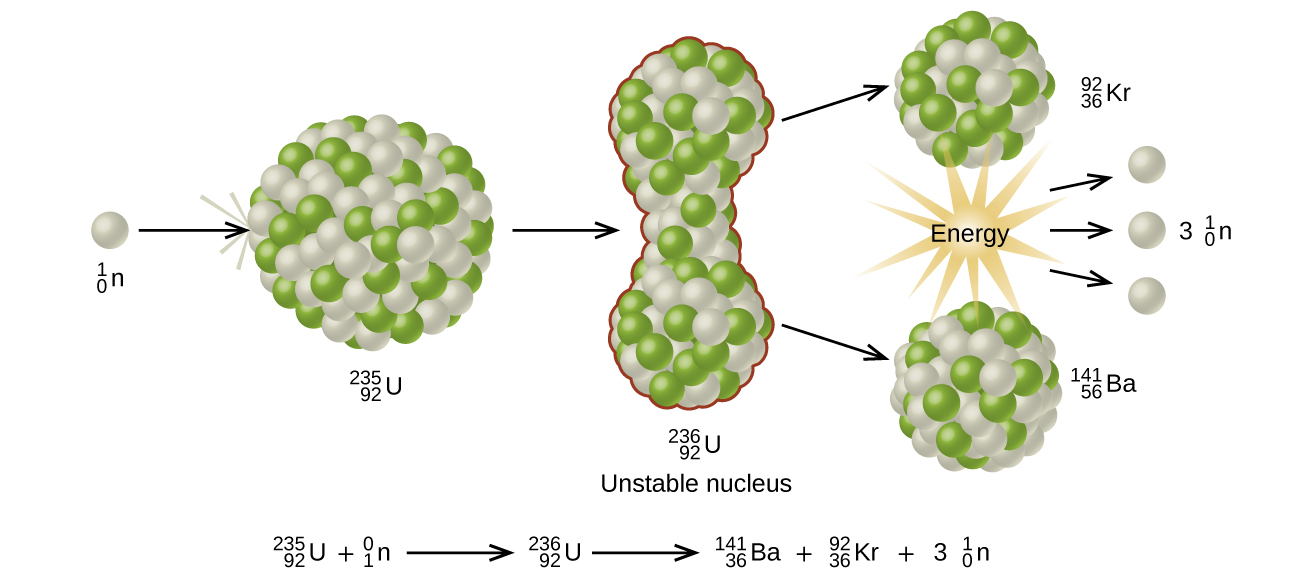
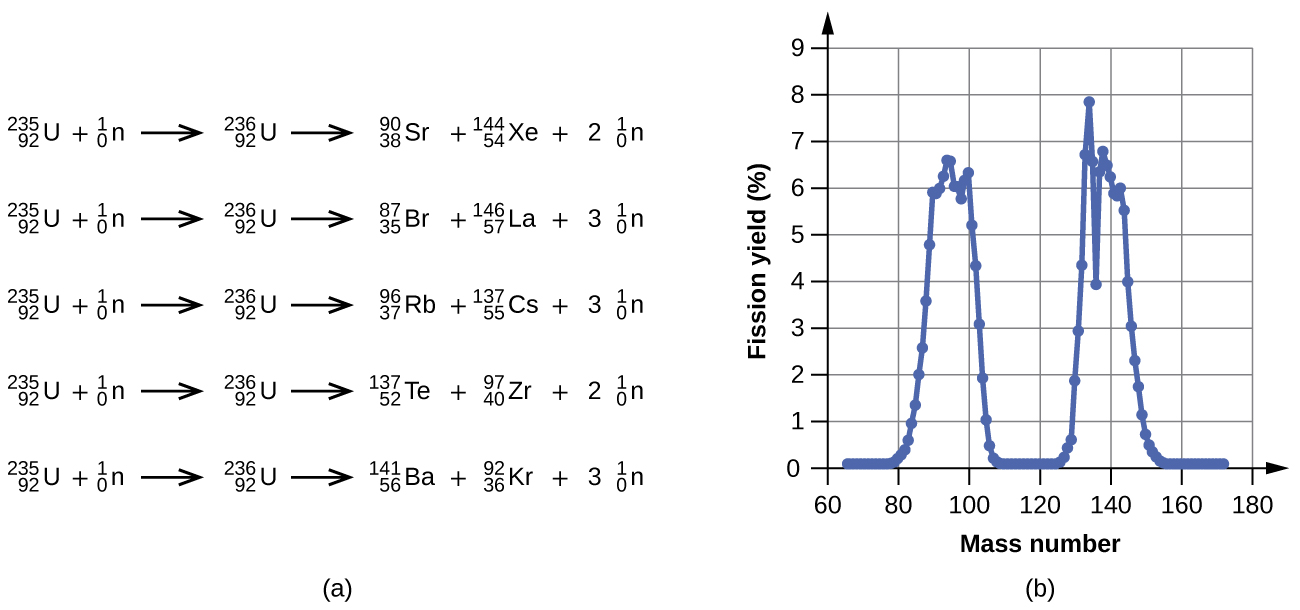
Among the products of Meitner, Hahn, and Strassman’s fission reaction were barium, krypton, lanthanum, and cerium, all of which have nuclei that are more stable than uranium-235. Since then, hundreds of different isotopes have been observed among the products of fissionable substances. A few of the many reactions that occur for U-235, and a graph showing the distribution of its fission products and their yields, are shown in (Figure). Similar fission reactions have been observed with other uranium isotopes, as well as with a variety of other isotopes such as those of plutonium.
View this link to see a simulation of nuclear fission.
A tremendous amount of energy is produced by the fission of heavy elements. For instance, when one mole of U-235 undergoes fission, the products weigh about 0.2 grams less than the reactants; this “lost” mass is converted into a very large amount of energy, about 1.8 \(×\) 1010 kJ per mole of U-235. Nuclear fission reactions produce incredibly large amounts of energy compared to chemical reactions. The fission of 1 kilogram of uranium-235, for example, produces about 2.5 million times as much energy as is produced by burning 1 kilogram of coal.
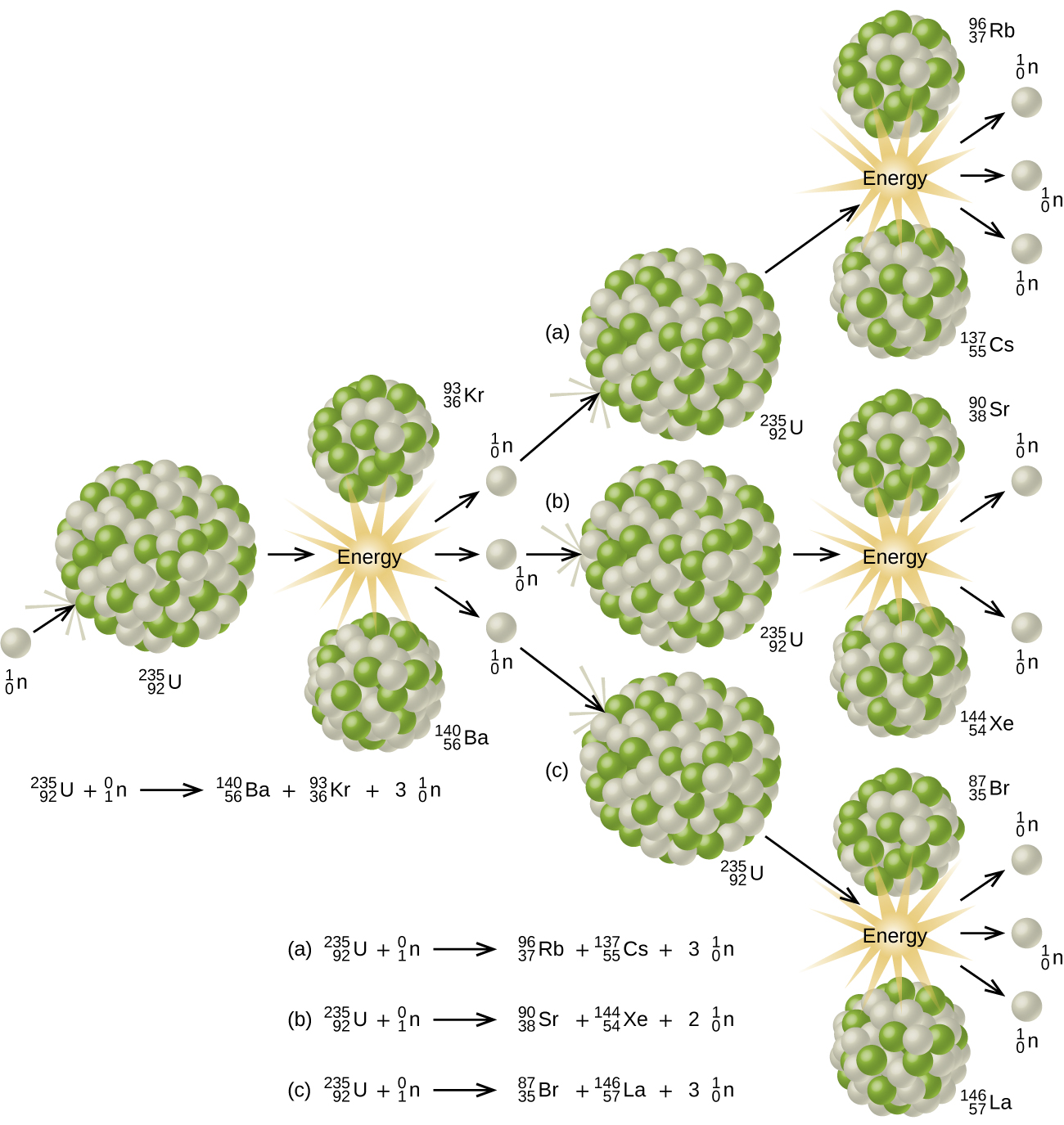
As described earlier, when undergoing fission U-235 produces two “medium-sized” nuclei, and two or three neutrons. These neutrons may then cause the fission of other uranium-235 atoms, which in turn provide more neutrons that can cause fission of even more nuclei, and so on. If this occurs, we have a nuclear chain reaction (see (Figure)). On the other hand, if too many neutrons escape the bulk material without interacting with a nucleus, then no chain reaction will occur.
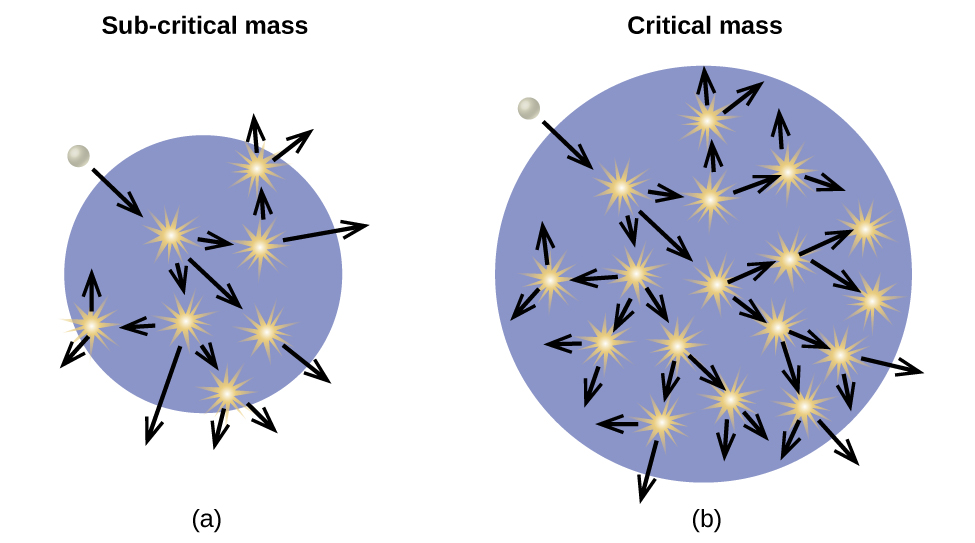
Material that can sustain a nuclear fission chain reaction is said to be fissile or fissionable. (Technically, fissile material can undergo fission with neutrons of any energy, whereas fissionable material requires high-energy neutrons.) Nuclear fission becomes self-sustaining when the number of neutrons produced by fission equals or exceeds the number of neutrons absorbed by splitting nuclei plus the number that escape into the surroundings. The amount of a fissionable material that will support a self-sustaining chain reaction is a critical mass. An amount of fissionable material that cannot sustain a chain reaction is a subcritical mass. An amount of material in which there is an increasing rate of fission is known as a supercritical mass. The critical mass depends on the type of material: its purity, the temperature, the shape of the sample, and how the neutron reactions are controlled ((Figure)).
An atomic bomb ((Figure)) contains several pounds of fissionable material, \({}_{\phantom{\rule{0.5em}{0ex}}92}^{235}\text{U}\) or \({}_{\phantom{\rule{0.5em}{0ex}}94}^{239}\text{Pu},\) a source of neutrons, and an explosive device for compressing it quickly into a small volume. When fissionable material is in small pieces, the proportion of neutrons that escape through the relatively large surface area is great, and a chain reaction does not take place. When the small pieces of fissionable material are brought together quickly to form a body with a mass larger than the critical mass, the relative number of escaping neutrons decreases, and a chain reaction and explosion result.
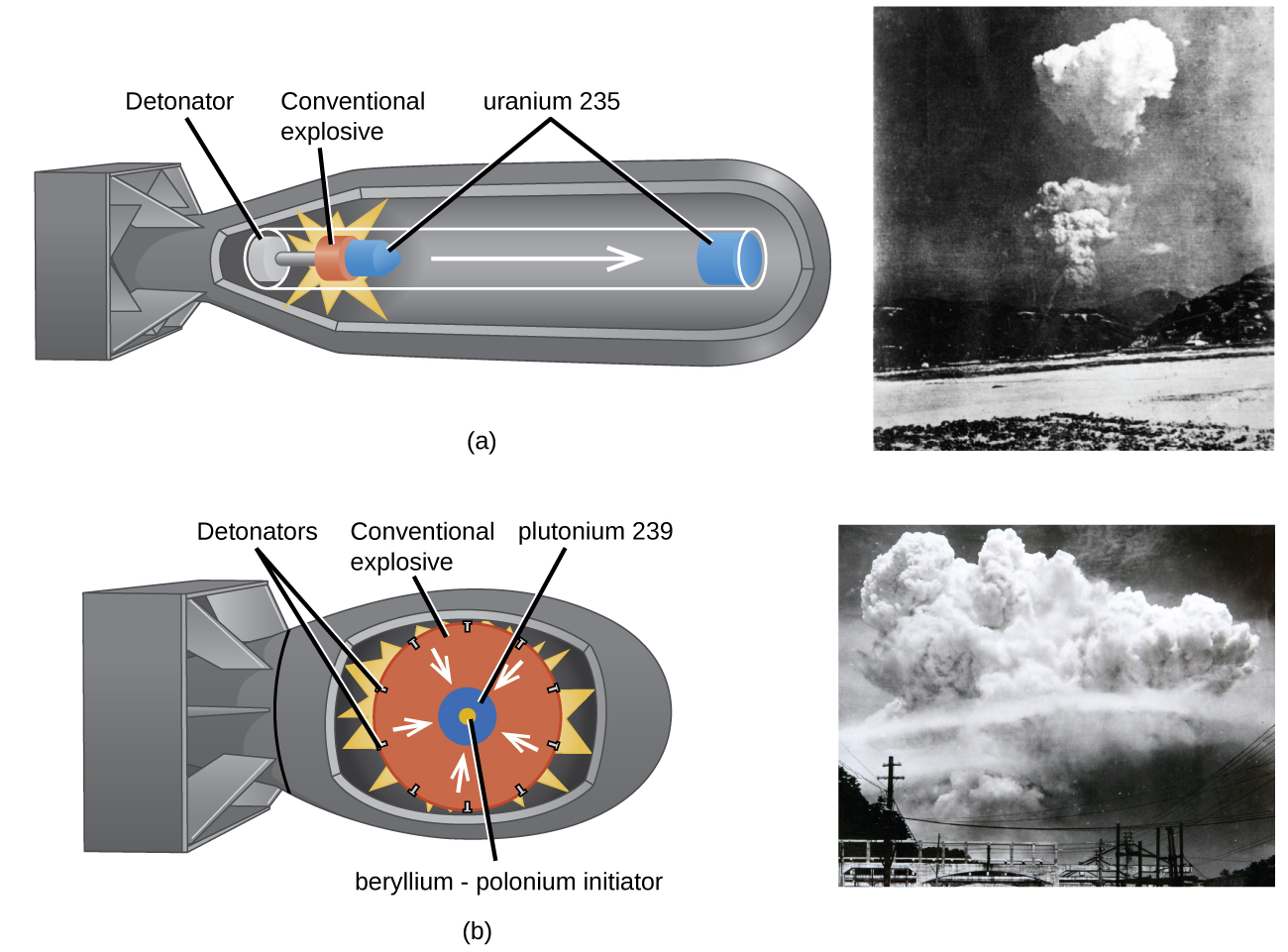
Fission Reactors
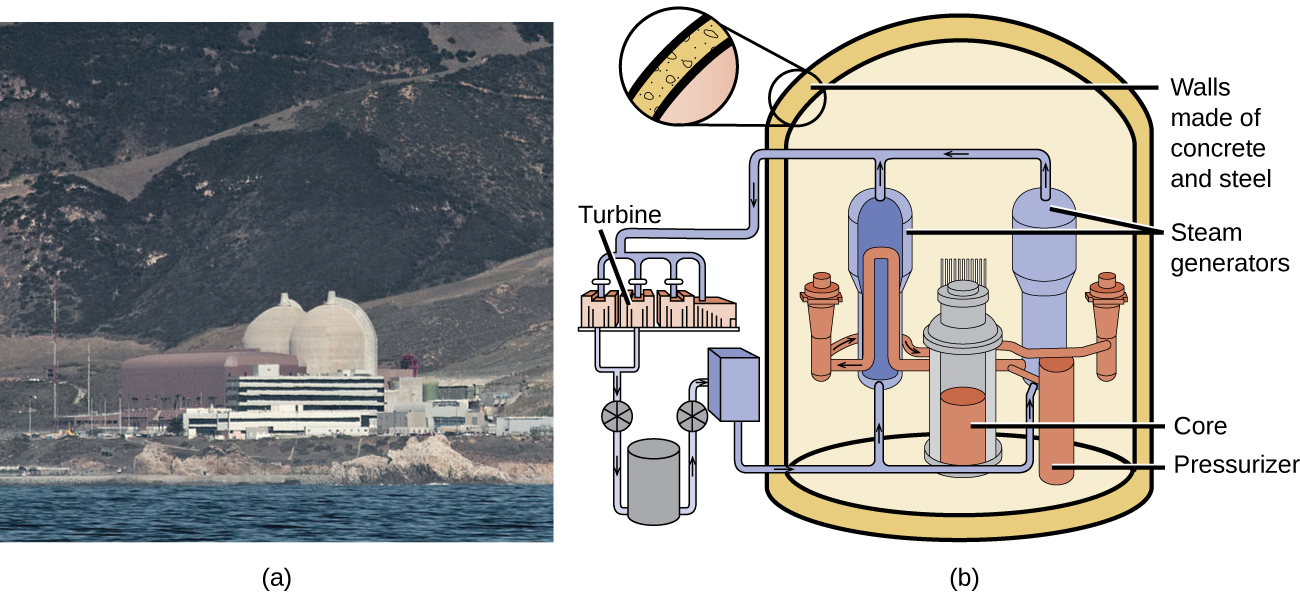
Chain reactions of fissionable materials can be controlled and sustained without an explosion in a nuclear reactor ((Figure)). Any nuclear reactor that produces power via the fission of uranium or plutonium by bombardment with neutrons must have at least five components: nuclear fuel consisting of fissionable material, a nuclear moderator, reactor coolant, control rods, and a shield and containment system. We will discuss these components in greater detail later in the section. The reactor works by separating the fissionable nuclear material such that a critical mass cannot be formed, controlling both the flux and absorption of neutrons to allow shutting down the fission reactions. In a nuclear reactor used for the production of electricity, the energy released by fission reactions is trapped as thermal energy and used to boil water and produce steam. The steam is used to turn a turbine, which powers a generator for the production of electricity.
Nuclear Fuels
Nuclear fuel consists of a fissionable isotope, such as uranium-235, which must be present in sufficient quantity to provide a self-sustaining chain reaction. In the United States, uranium ores contain from 0.05–0.3% of the uranium oxide U3O8; the uranium in the ore is about 99.3% nonfissionable U-238 with only 0.7% fissionable U-235. Nuclear reactors require a fuel with a higher concentration of U-235 than is found in nature; it is normally enriched to have about 5% of uranium mass as U-235. At this concentration, it is not possible to achieve the supercritical mass necessary for a nuclear explosion. Uranium can be enriched by gaseous diffusion (the only method currently used in the US), using a gas centrifuge, or by laser separation.
In the gaseous diffusion enrichment plant where U-235 fuel is prepared, UF6 (uranium hexafluoride) gas at low pressure moves through barriers that have holes just barely large enough for UF6 to pass through. The slightly lighter 235UF6 molecules diffuse through the barrier slightly faster than the heavier 238UF6 molecules. This process is repeated through hundreds of barriers, gradually increasing the concentration of 235UF6 to the level needed by the nuclear reactor. The basis for this process, Graham’s law, is described in the chapter on gases. The enriched UF6 gas is collected, cooled until it solidifies, and then taken to a fabrication facility where it is made into fuel assemblies. Each fuel assembly consists of fuel rods that contain many thimble-sized, ceramic-encased, enriched uranium (usually UO2) fuel pellets. Modern nuclear reactors may contain as many as 10 million fuel pellets. The amount of energy in each of these pellets is equal to that in almost a ton of coal or 150 gallons of oil.
Nuclear Moderators
Neutrons produced by nuclear reactions move too fast to cause fission (refer back to (Figure)). They must first be slowed to be absorbed by the fuel and produce additional nuclear reactions. A nuclear moderator is a substance that slows the neutrons to a speed that is low enough to cause fission. Early reactors used high-purity graphite as a moderator. Modern reactors in the US exclusively use heavy water \(\left({{}_{1}^{2}\text{H}}_{2}\text{O}\right)\) or light water (ordinary H2O), whereas some reactors in other countries use other materials, such as carbon dioxide, beryllium, or graphite.
Reactor Coolants
A nuclear reactor coolant is used to carry the heat produced by the fission reaction to an external boiler and turbine, where it is transformed into electricity. Two overlapping coolant loops are often used; this counteracts the transfer of radioactivity from the reactor to the primary coolant loop. All nuclear power plants in the US use water as a coolant. Other coolants include molten sodium, lead, a lead-bismuth mixture, or molten salts.
Control Rods
Nuclear reactors use control rods ((Figure)) to control the fission rate of the nuclear fuel by adjusting the number of slow neutrons present to keep the rate of the chain reaction at a safe level. Control rods are made of boron, cadmium, hafnium, or other elements that are able to absorb neutrons. Boron-10, for example, absorbs neutrons by a reaction that produces lithium-7 and alpha particles:
When control rod assemblies are inserted into the fuel element in the reactor core, they absorb a larger fraction of the slow neutrons, thereby slowing the rate of the fission reaction and decreasing the power produced. Conversely, if the control rods are removed, fewer neutrons are absorbed, and the fission rate and energy production increase. In an emergency, the chain reaction can be shut down by fully inserting all of the control rods into the nuclear core between the fuel rods.
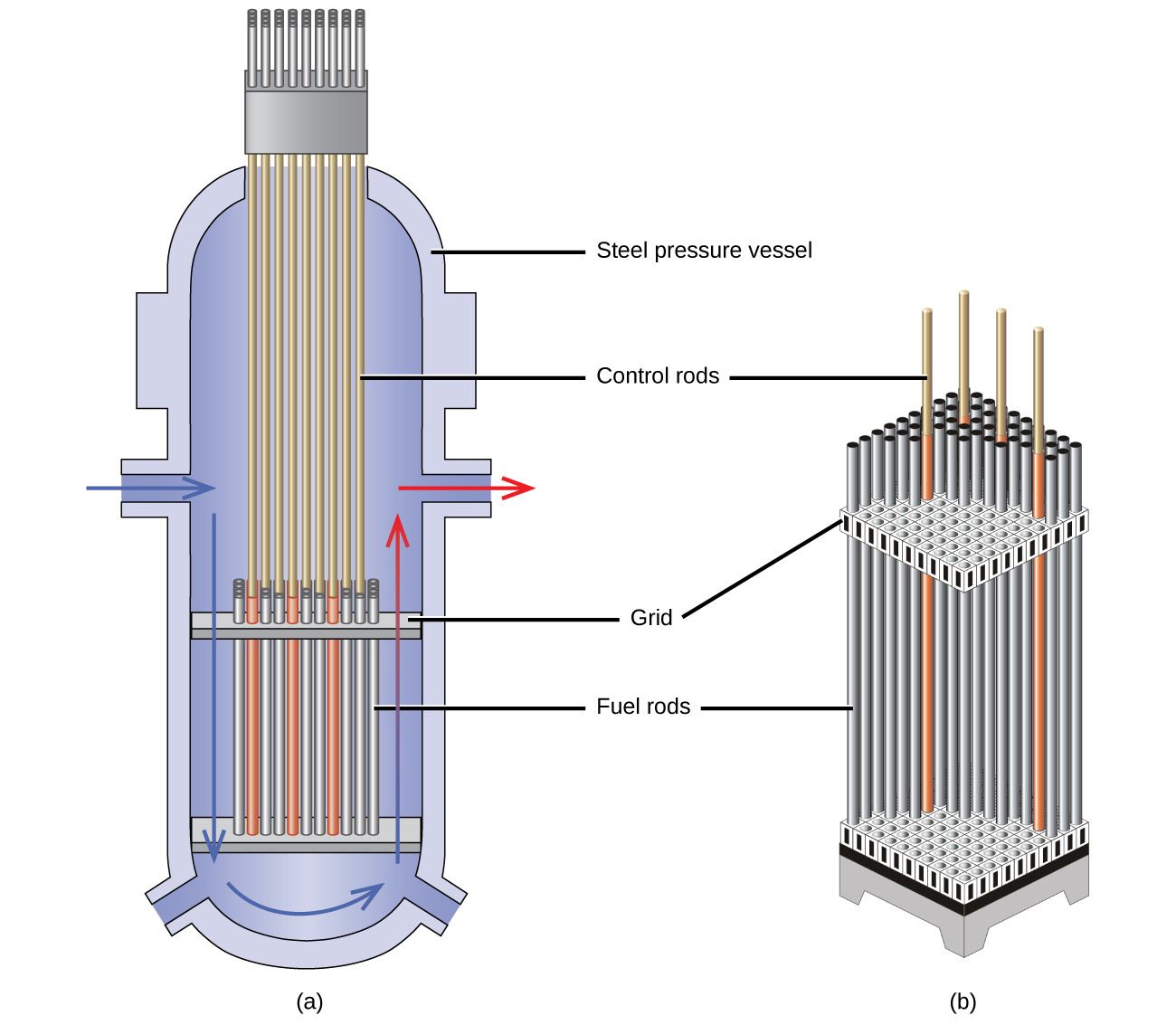
Shield and Containment System
During its operation, a nuclear reactor produces neutrons and other radiation. Even when shut down, the decay products are radioactive. In addition, an operating reactor is thermally very hot, and high pressures result from the circulation of water or another coolant through it. Thus, a reactor must withstand high temperatures and pressures, and must protect operating personnel from the radiation. Reactors are equipped with a containment system (or shield) that consists of three parts:
- The reactor vessel, a steel shell that is 3–20-centimeters thick and, with the moderator, absorbs much of the radiation produced by the reactor
- A main shield of 1–3 meters of high-density concrete
- A personnel shield of lighter materials that protects operators from γ rays and X-rays
In addition, reactors are often covered with a steel or concrete dome that is designed to contain any radioactive materials might be released by a reactor accident.
Click here to watch a 3-minute video from the Nuclear Energy Institute on how nuclear reactors work.
Nuclear power plants are designed in such a way that they cannot form a supercritical mass of fissionable material and therefore cannot create a nuclear explosion. But as history has shown, failures of systems and safeguards can cause catastrophic accidents, including chemical explosions and nuclear meltdowns (damage to the reactor core from overheating). The following Chemistry in Everyday Life feature explores three infamous meltdown incidents.
The importance of cooling and containment are amply illustrated by three major accidents that occurred with the nuclear reactors at nuclear power generating stations in the United States (Three Mile Island), the former Soviet Union (Chernobyl), and Japan (Fukushima).
In March 1979, the cooling system of the Unit 2 reactor at Three Mile Island Nuclear Generating Station in Pennsylvania failed, and the cooling water spilled from the reactor onto the floor of the containment building. After the pumps stopped, the reactors overheated due to the high radioactive decay heat produced in the first few days after the nuclear reactor shut down. The temperature of the core climbed to at least 2200 °C, and the upper portion of the core began to melt. In addition, the zirconium alloy cladding of the fuel rods began to react with steam and produced hydrogen:
The hydrogen accumulated in the confinement building, and it was feared that there was danger of an explosion of the mixture of hydrogen and air in the building. Consequently, hydrogen gas and radioactive gases (primarily krypton and xenon) were vented from the building. Within a week, cooling water circulation was restored and the core began to cool. The plant was closed for nearly 10 years during the cleanup process.
Although zero discharge of radioactive material is desirable, the discharge of radioactive krypton and xenon, such as occurred at the Three Mile Island plant, is among the most tolerable. These gases readily disperse in the atmosphere and thus do not produce highly radioactive areas. Moreover, they are noble gases and are not incorporated into plant and animal matter in the food chain. Effectively none of the heavy elements of the core of the reactor were released into the environment, and no cleanup of the area outside of the containment building was necessary ((Figure)).

Another major nuclear accident involving a reactor occurred in April 1986, at the Chernobyl Nuclear Power Plant in Ukraine, which was still a part of the former Soviet Union. While operating at low power during an unauthorized experiment with some of its safety devices shut off, one of the reactors at the plant became unstable. Its chain reaction became uncontrollable and increased to a level far beyond what the reactor was designed for. The steam pressure in the reactor rose to between 100 and 500 times the full power pressure and ruptured the reactor. Because the reactor was not enclosed in a containment building, a large amount of radioactive material spewed out, and additional fission products were released, as the graphite (carbon) moderator of the core ignited and burned. The fire was controlled, but over 200 plant workers and firefighters developed acute radiation sickness and at least 32 soon died from the effects of the radiation. It is predicted that about 4000 more deaths will occur among emergency workers and former Chernobyl residents from radiation-induced cancer and leukemia. The reactor has since been encapsulated in steel and concrete, a now-decaying structure known as the sarcophagus. Almost 30 years later, significant radiation problems still persist in the area, and Chernobyl largely remains a wasteland.
In 2011, the Fukushima Daiichi Nuclear Power Plant in Japan was badly damaged by a 9.0-magnitude earthquake and resulting tsunami. Three reactors up and running at the time were shut down automatically, and emergency generators came online to power electronics and coolant systems. However, the tsunami quickly flooded the emergency generators and cut power to the pumps that circulated coolant water through the reactors. High-temperature steam in the reactors reacted with zirconium alloy to produce hydrogen gas. The gas escaped into the containment building, and the mixture of hydrogen and air exploded. Radioactive material was released from the containment vessels as the result of deliberate venting to reduce the hydrogen pressure, deliberate discharge of coolant water into the sea, and accidental or uncontrolled events.
An evacuation zone around the damaged plant extended over 12.4 miles away, and an estimated 200,000 people were evacuated from the area. All 48 of Japan’s nuclear power plants were subsequently shut down, remaining shuttered as of December 2014. Since the disaster, public opinion has shifted from largely favoring to largely opposing increasing the use of nuclear power plants, and a restart of Japan’s atomic energy program is still stalled ((Figure)).
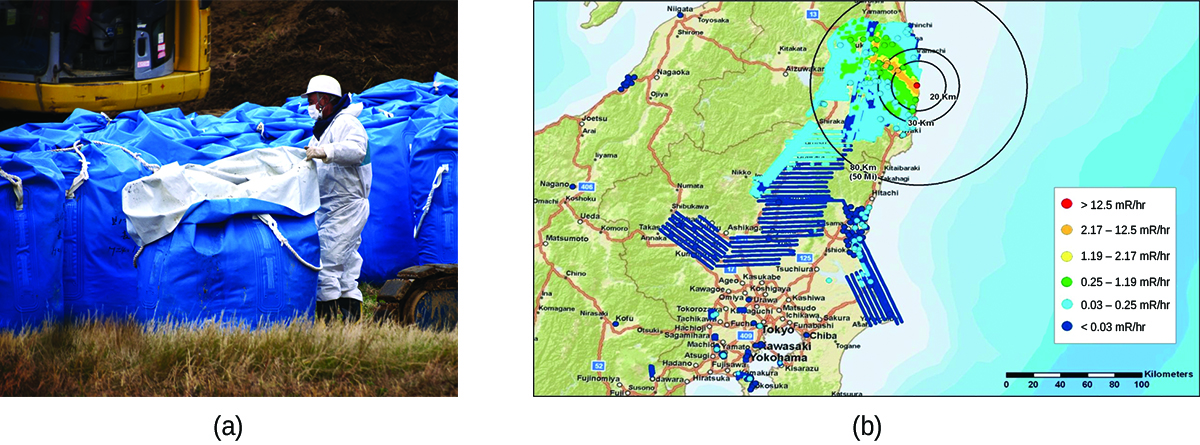
The energy produced by a reactor fueled with enriched uranium results from the fission of uranium as well as from the fission of plutonium produced as the reactor operates. As discussed previously, the plutonium forms from the combination of neutrons and the uranium in the fuel. In any nuclear reactor, only about 0.1% of the mass of the fuel is converted into energy. The other 99.9% remains in the fuel rods as fission products and unused fuel. All of the fission products absorb neutrons, and after a period of several months to a few years, depending on the reactor, the fission products must be removed by changing the fuel rods. Otherwise, the concentration of these fission products would increase and absorb more neutrons until the reactor could no longer operate.
Spent fuel rods contain a variety of products, consisting of unstable nuclei ranging in atomic number from 25 to 60, some transuranium elements, including plutonium and americium, and unreacted uranium isotopes. The unstable nuclei and the transuranium isotopes give the spent fuel a dangerously high level of radioactivity. The long-lived isotopes require thousands of years to decay to a safe level. The ultimate fate of the nuclear reactor as a significant source of energy in the United States probably rests on whether or not a politically and scientifically satisfactory technique for processing and storing the components of spent fuel rods can be developed.
Explore the information in this link to learn about the approaches to nuclear waste management.
Nuclear Fusion and Fusion Reactors
The process of converting very light nuclei into heavier nuclei is also accompanied by the conversion of mass into large amounts of energy, a process called fusion. The principal source of energy in the sun is a net fusion reaction in which four hydrogen nuclei fuse and produce one helium nucleus and two positrons. This is a net reaction of a more complicated series of events:
A helium nucleus has a mass that is 0.7% less than that of four hydrogen nuclei; this lost mass is converted into energy during the fusion. This reaction produces about 3.6 \(×\) 1011 kJ of energy per mole of \({}_{2}^{4}\text{He}\) produced. This is somewhat larger than the energy produced by the nuclear fission of one mole of U-235 (1.8 \(×\) 1010 kJ), and over 3 million times larger than the energy produced by the (chemical) combustion of one mole of octane (5471 kJ).
It has been determined that the nuclei of the heavy isotopes of hydrogen, a deuteron, \({}_{1}^{2}\) and a triton, \({}_{1}^{3},\) undergo fusion at extremely high temperatures (thermonuclear fusion). They form a helium nucleus and a neutron:
This change proceeds with a mass loss of 0.0188 amu, corresponding to the release of 1.69 \(×\) 109 kilojoules per mole of \({}_{2}^{4}\text{He}\) formed. The very high temperature is necessary to give the nuclei enough kinetic energy to overcome the very strong repulsive forces resulting from the positive charges on their nuclei so they can collide.
Useful fusion reactions require very high temperatures for their initiation—about 15,000,000 K or more. At these temperatures, all molecules dissociate into atoms, and the atoms ionize, forming plasma. These conditions occur in an extremely large number of locations throughout the universe—stars are powered by fusion. Humans have already figured out how to create temperatures high enough to achieve fusion on a large scale in thermonuclear weapons. A thermonuclear weapon such as a hydrogen bomb contains a nuclear fission bomb that, when exploded, gives off enough energy to produce the extremely high temperatures necessary for fusion to occur.
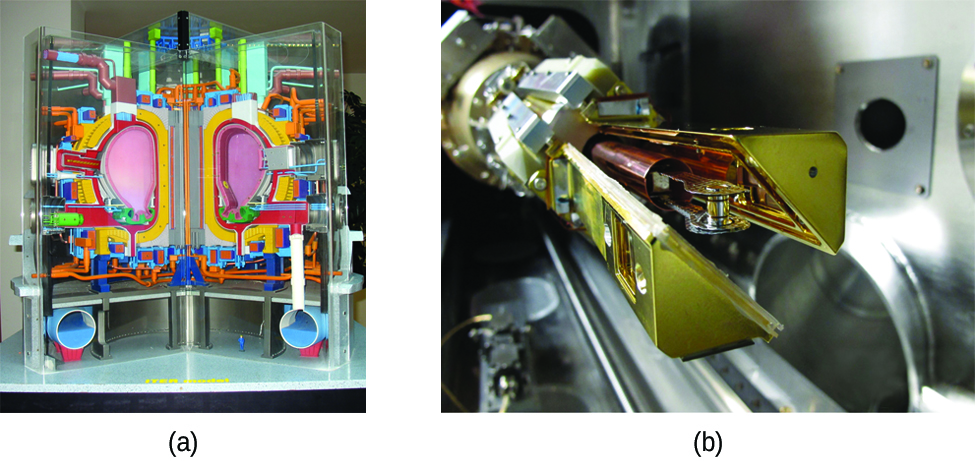
Another much more beneficial way to create fusion reactions is in a fusion reactor, a nuclear reactor in which fusion reactions of light nuclei are controlled. Because no solid materials are stable at such high temperatures, mechanical devices cannot contain the plasma in which fusion reactions occur. Two techniques to contain plasma at the density and temperature necessary for a fusion reaction are currently the focus of intensive research efforts: containment by a magnetic field and by the use of focused laser beams ((Figure)). A number of large projects are working to attain one of the biggest goals in science: getting hydrogen fuel to ignite and produce more energy than the amount supplied to achieve the extremely high temperatures and pressures that are required for fusion. At the time of this writing, there are no self-sustaining fusion reactors operating in the world, although small-scale controlled fusion reactions have been run for very brief periods.
Key Concepts and Summary
It is possible to produce new atoms by bombarding other atoms with nuclei or high-speed particles. The products of these transmutation reactions can be stable or radioactive. A number of artificial elements, including technetium, astatine, and the transuranium elements, have been produced in this way.
Nuclear power as well as nuclear weapon detonations can be generated through fission (reactions in which a heavy nucleus is split into two or more lighter nuclei and several neutrons). Because the neutrons may induce additional fission reactions when they combine with other heavy nuclei, a chain reaction can result. Useful power is obtained if the fission process is carried out in a nuclear reactor. The conversion of light nuclei into heavier nuclei (fusion) also produces energy. At present, this energy has not been contained adequately and is too expensive to be feasible for commercial energy production.
Chemistry End of Chapter Exercises
Write the balanced nuclear equation for the production of the following transuranium elements:
(a) berkelium-244, made by the reaction of Am-241 and He-4
(b) fermium-254, made by the reaction of Pu-239 with a large number of neutrons
(c) lawrencium-257, made by the reaction of Cf-250 and B-11
(d) dubnium-260, made by the reaction of Cf-249 and N-15
(a) \({}_{\phantom{\rule{0.5em}{0ex}}95}^{241}\text{Am}+{}_{2}^{4}\text{He}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{\phantom{\rule{0.5em}{0ex}}97}^{244}\text{Bk}+{}_{0}^{1}\text{n};\) (b) \({}_{\phantom{\rule{0.5em}{0ex}}94}^{239}\text{Pu}+15{}_{0}^{1}\text{n}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{100}^{254}\text{Fm}+6{}_{-1}^{\phantom{\rule{0.5em}{0ex}}0}\text{e};\) (c) \({}_{\phantom{\rule{0.5em}{0ex}}98}^{250}\text{Cf}+{}_{\phantom{\rule{0.5em}{0ex}}5}^{11}\text{B}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{103}^{257}\text{Lr}+4{}_{0}^{1}\text{n};\) (d) \({}_{\phantom{\rule{0.5em}{0ex}}98}^{249}\text{Cf}+{}_{\phantom{\rule{0.5em}{0ex}}7}^{15}\text{N}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{105}^{260}\text{Db}+4{}_{0}^{1}\text{n}\)
How does nuclear fission differ from nuclear fusion? Why are both of these processes exothermic?
Both fusion and fission are nuclear reactions. Why is a very high temperature required for fusion, but not for fission?
Two nuclei must collide for fusion to occur. High temperatures are required to give the nuclei enough kinetic energy to overcome the very strong repulsion resulting from their positive charges.
Cite the conditions necessary for a nuclear chain reaction to take place. Explain how it can be controlled to produce energy, but not produce an explosion.
Describe the components of a nuclear reactor.
A nuclear reactor consists of the following:
- A nuclear fuel. A fissionable isotope must be present in large enough quantities to sustain a controlled chain reaction. The radioactive isotope is contained in tubes called fuel rods.
- A moderator. A moderator slows neutrons produced by nuclear reactions so that they can be absorbed by the fuel and cause additional nuclear reactions.
- A coolant. The coolant carries heat from the fission reaction to an external boiler and turbine where it is transformed into electricity.
- A control system. The control system consists of control rods placed between fuel rods to absorb neutrons and is used to adjust the number of neutrons and keep the rate of the chain reaction at a safe level.
- A shield and containment system. The function of this component is to protect workers from radiation produced by the nuclear reactions and to withstand the high pressures resulting from high-temperature reactions.
In usual practice, both a moderator and control rods are necessary to operate a nuclear chain reaction safely for the purpose of energy production. Cite the function of each and explain why both are necessary.
Describe how the potential energy of uranium is converted into electrical energy in a nuclear power plant.
The fission of uranium generates heat, which is carried to an external steam generator (boiler). The resulting steam turns a turbine that powers an electrical generator.
The mass of a hydrogen atom \(\left({}_{1}^{1}\text{H}\right)\) is 1.007825 amu; that of a tritium atom \(\left({}_{1}^{3}\text{H}\right)\) is 3.01605 amu; and that of an α particle is 4.00150 amu. How much energy in kilojoules per mole of \({}_{2}^{4}\text{He}\) produced is released by the following fusion reaction: \({}_{1}^{1}\text{H}+{}_{1}^{3}\text{H}\phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}{}_{2}^{4}\text{He}.\)
Glossary
- chain reaction
- repeated fission caused when the neutrons released in fission bombard other atoms
- containment system
- (also, shield) a three-part structure of materials that protects the exterior of a nuclear fission reactor and operating personnel from the high temperatures, pressures, and radiation levels inside the reactor
- control rod
- material inserted into the fuel assembly that absorbs neutrons and can be raised or lowered to adjust the rate of a fission reaction
- critical mass
- amount of fissionable material that will support a self-sustaining (nuclear fission) chain reaction
- fissile (or fissionable)
- when a material is capable of sustaining a nuclear fission reaction
- fission
- splitting of a heavier nucleus into two or more lighter nuclei, usually accompanied by the conversion of mass into large amounts of energy
- fusion
- combination of very light nuclei into heavier nuclei, accompanied by the conversion of mass into large amounts of energy
- fusion reactor
- nuclear reactor in which fusion reactions of light nuclei are controlled
- nuclear fuel
- fissionable isotope present in sufficient quantities to provide a self-sustaining chain reaction in a nuclear reactor
- nuclear moderator
- substance that slows neutrons to a speed low enough to cause fission
- nuclear reactor
- environment that produces energy via nuclear fission in which the chain reaction is controlled and sustained without explosion
- nuclear transmutation
- conversion of one nuclide into another nuclide
- particle accelerator
- device that uses electric and magnetic fields to increase the kinetic energy of nuclei used in transmutation reactions
- reactor coolant
- assembly used to carry the heat produced by fission in a reactor to an external boiler and turbine where it is transformed into electricity
- subcritical mass
- amount of fissionable material that cannot sustain a chain reaction; less than a critical mass
- supercritical mass
- amount of material in which there is an increasing rate of fission
- transmutation reaction
- bombardment of one type of nuclei with other nuclei or neutrons
- transuranium element
- element with an atomic number greater than 92; these elements do not occur in nature

