83 Relative Strengths of Acids and Bases
[latexpage]
Learning Objectives
By the end of this section, you will be able to:
- Assess the relative strengths of acids and bases according to their ionization constants
- Rationalize trends in acid–base strength in relation to molecular structure
- Carry out equilibrium calculations for weak acid–base systems
Acid and Base Ionization Constants
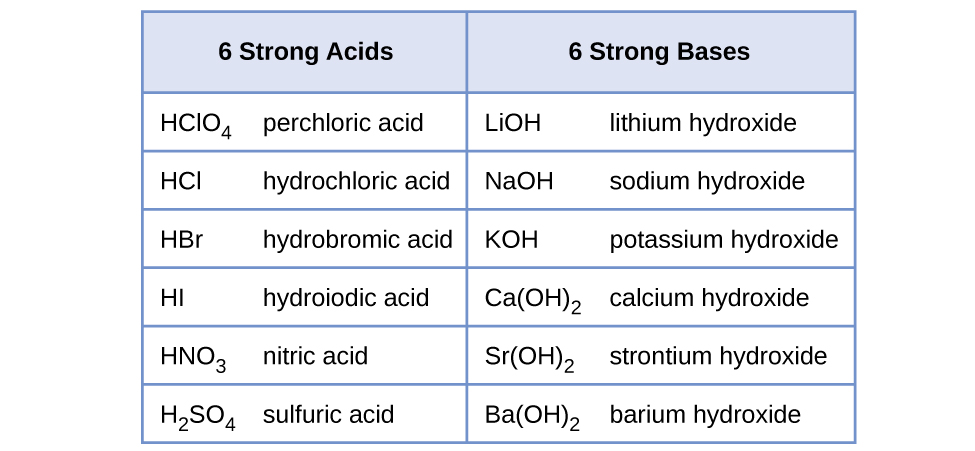
The relative strength of an acid or base is the extent to which it ionizes when dissolved in water. If the ionization reaction is essentially complete, the acid or base is termed strong; if relatively little ionization occurs, the acid or base is weak. As will be evident throughout the remainder of this chapter, there are many more weak acids and bases than strong ones. The most common strong acids and bases are listed in (Figure).
The relative strengths of acids may be quantified by measuring their equilibrium constants in aqueous solutions. In solutions of the same concentration, stronger acids ionize to a greater extent, and so yield higher concentrations of hydronium ions than do weaker acids. The equilibrium constant for an acid is called the acid-ionization constant, Ka. For the reaction of an acid HA:
the acid ionization constant is written
where the concentrations are those at equilibrium. Although water is a reactant in the reaction, it is the solvent as well, so we do not include [H2O] in the equation. The larger the Ka of an acid, the larger the concentration of \({\text{H}}_{3}{\text{O}}^{\text{+}}\) and A− relative to the concentration of the nonionized acid, HA, in an equilibrium mixture, and the stronger the acid. An acid is classified as “strong” when it undergoes complete ionization, in which case the concentration of HA is zero and the acid ionization constant is immeasurably large (Ka ≈ ∞). Acids that are partially ionized are called “weak,” and their acid ionization constants may be experimentally measured. A table of ionization constants for weak acids is provided in Appendix H.
To illustrate this idea, three acid ionization equations and Ka values are shown below. The ionization constants increase from first to last of the listed equations, indicating the relative acid strength increases in the order CH3CO2H < HNO2 < \({\text{HSO}}_{4}{}^{\text{−}}:\)
Another measure of the strength of an acid is its percent ionization. The percent ionization of a weak acid is defined in terms of the composition of an equilibrium mixture:
where the numerator is equivalent to the concentration of the acid’s conjugate base (per stoichiometry, [A−] = [H3O+]). Unlike the Ka value, the percent ionization of a weak acid varies with the initial concentration of acid, typically decreasing as concentration increases. Equilibrium calculations of the sort described later in this chapter can be used to confirm this behavior.
Calculation of Percent Ionization from pH Calculate the percent ionization of a 0.125-M solution of nitrous acid (a weak acid), with a pH of 2.09.
Solution The percent ionization for an acid is:
Converting the provided pH to hydronium ion molarity yields
Substituting this value and the provided initial acid concentration into the percent ionization equation gives
(Recall the provided pH value of 2.09 is logarithmic, and so it contains just two significant digits, limiting the certainty of the computed percent ionization.)
Check Your Learning Calculate the percent ionization of a 0.10-M solution of acetic acid with a pH of 2.89.
1.3% ionized
View the simulation of strong and weak acids and bases at the molecular level.
Just as for acids, the relative strength of a base is reflected in the magnitude of its base-ionization constant (Kb) in aqueous solutions. In solutions of the same concentration, stronger bases ionize to a greater extent, and so yield higher hydroxide ion concentrations than do weaker bases. A stronger base has a larger ionization constant than does a weaker base. For the reaction of a base, B:
the ionization constant is written as
Inspection of the data for three weak bases presented below shows the base strength increases in the order \({\text{NO}}_{2}{}^{-}<{\text{CH}}_{2}{\text{CO}}_{2}{}^{-}<{\text{NH}}_{3}.\)
A table of ionization constants for weak bases appears in Appendix I. As for acids, the relative strength of a base is also reflected in its percent ionization, computed as
but will vary depending on the base ionization constant and the initial concentration of the solution.
Relative Strengths of Conjugate Acid-Base Pairs
Brønsted-Lowry acid-base chemistry is the transfer of protons; thus, logic suggests a relation between the relative strengths of conjugate acid-base pairs. The strength of an acid or base is quantified in its ionization constant, Ka or Kb, which represents the extent of the acid or base ionization reaction. For the conjugate acid-base pair HA / A−, ionization equilibrium equations and ionization constant expressions are
Adding these two chemical equations yields the equation for the autoionization for water:
As discussed in another chapter on equilibrium, the equilibrium constant for a summed reaction is equal to the mathematical product of the equilibrium constants for the added reactions, and so
This equation states the relation between ionization constants for any conjugate acid-base pair, namely, their mathematical product is equal to the ion product of water, Kw. By rearranging this equation, a reciprocal relation between the strengths of a conjugate acid-base pair becomes evident:
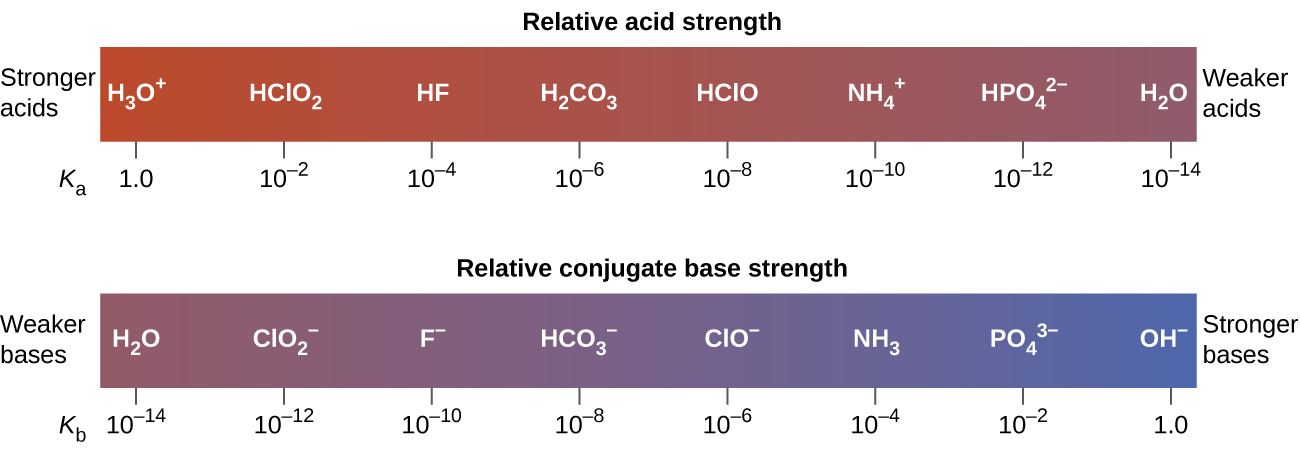
The inverse proportional relation between Ka and Kb means the stronger the acid or base, the weaker its conjugate partner. (Figure) illustrates this relation for several conjugate acid-base pairs.
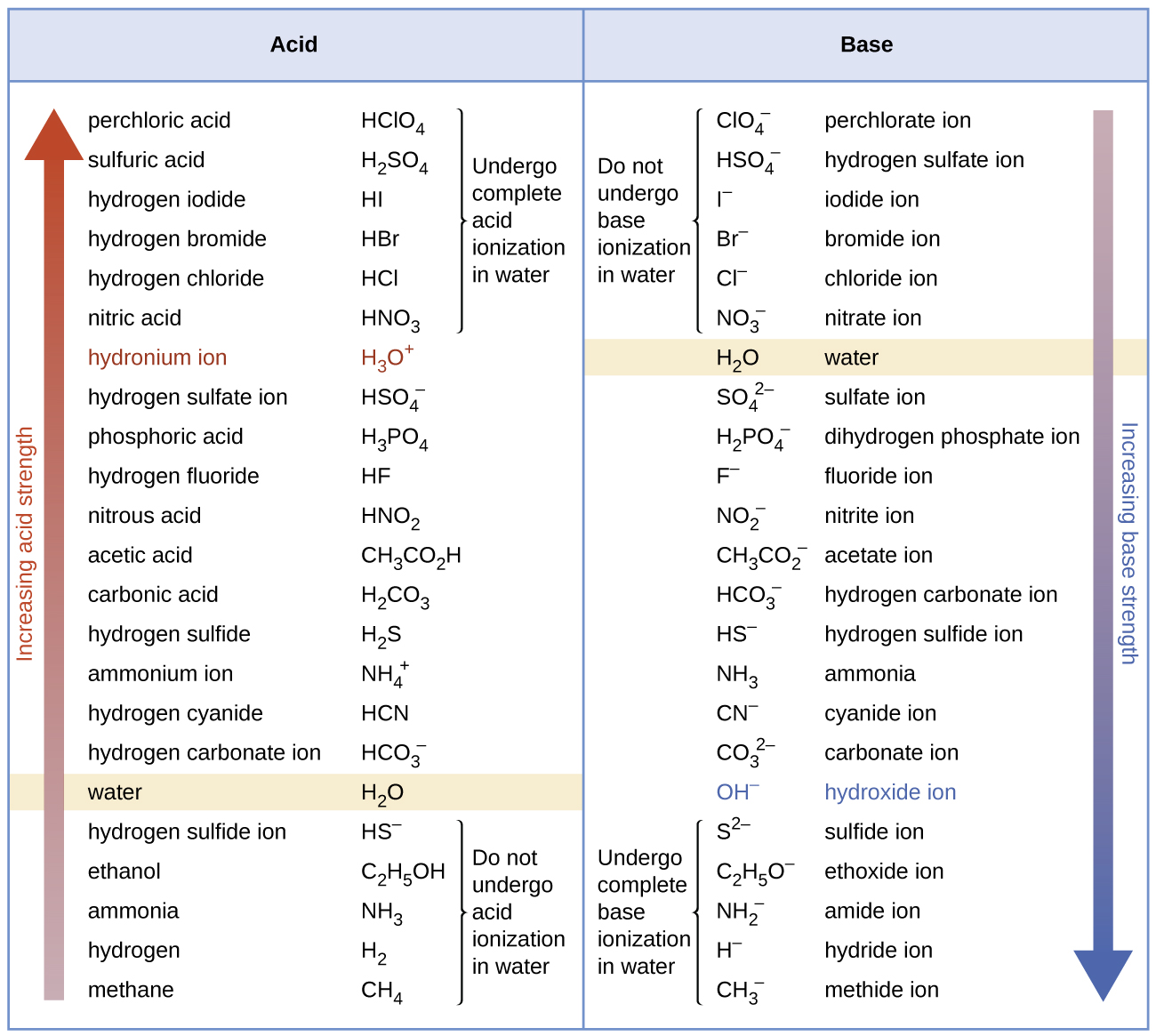
The listing of conjugate acid–base pairs shown in (Figure) is arranged to show the relative strength of each species as compared with water, whose entries are highlighted in each of the table’s columns. In the acid column, those species listed below water are weaker acids than water. These species do not undergo acid ionization in water; they are not Bronsted-Lowry acids. All the species listed above water are stronger acids, transferring protons to water to some extent when dissolved in an aqueous solution to generate hydronium ions. Species above water but below hydronium ion are weak acids, undergoing partial acid ionization, wheres those above hydronium ion are strong acids that are completely ionized in aqueous solution.
If all these strong acids are completely ionized in water, why does the column indicate they vary in strength, with nitric acid being the weakest and perchloric acid the strongest? Notice that the sole acid species present in an aqueous solution of any strong acid is H3O+(aq), meaning that hydronium ion is the strongest acid that may exist in water; any stronger acid will react completely with water to generate hydronium ions. This limit on the acid strength of solutes in a solution is called a leveling effect. To measure the differences in acid strength for “strong” acids, the acids must be dissolved in a solvent that is less basic than water. In such solvents, the acids will be “weak,” and so any differences in the extent of their ionization can be determined. For example, the binary hydrogen halides HCl, HBr, and HI are strong acids in water but weak acids in ethanol (strength increasing HCl < HBr < HI).
The right column of (Figure) lists a number of substances in order of increasing base strength from top to bottom. Following the same logic as for the left column, species listed above water are weaker bases and so they don’t undergo base ionization when dissolved in water. Species listed between water and its conjugate base, hydroxide ion, are weak bases that partially ionize. Species listed below hydroxide ion are strong bases that completely ionize in water to yield hydroxide ions (i.e., they are leveled to hydroxide). A comparison of the acid and base columns in this table supports the reciprocal relation between the strengths of conjugate acid-base pairs. For example, the conjugate bases of the strong acids (top of table) are all of negligible strength. A strong acid exhibits an immeasurably large Ka, and so its conjugate base will exhibit a Kb that is essentially zero:
\(\begin{array}{l}\text{strong}\phantom{\rule{0.2em}{0ex}}\text{acid}:\phantom{\rule{5em}{0ex}}{K}_{\text{a}}\approx \infty \\ \text{conjugate}\phantom{\rule{0.2em}{0ex}}\text{base}:\phantom{\rule{0.2em}{0ex}}{K}_{\text{b}}={K}_{\text{w}}\text{/}{K}_{\text{a}}={K}_{\text{w}}\text{/}\infty \approx 0\end{array}\)
A similar approach can be used to support the observation that conjugate acids of strong bases (Kb ≈ ∞) are of negligible strength (Ka ≈ 0).
Calculating Ionization Constants for Conjugate Acid-Base Pairs Use the Kb for the nitrite ion, \({\text{NO}}_{2}{}^{\text{−}},\) to calculate the Ka for its conjugate acid.
SolutionKb for \({\text{NO}}_{2}{}^{\text{−}}\) is given in this section as 2.17 \(×\) 10−11. The conjugate acid of \({\text{NO}}_{2}{}^{\text{−}}\) is HNO2; Ka for HNO2 can be calculated using the relationship:
Solving for Ka yields
This answer can be verified by finding the Ka for HNO2 in Appendix H.
Check Your LearningDetermine the relative acid strengths of \({\text{NH}}_{4}{}^{\text{+}}\) and HCN by comparing their ionization constants. The ionization constant of HCN is given in Appendix H as 4.9 \(×\) 10−10. The ionization constant of \({\text{NH}}_{4}{}^{\text{+}}\) is not listed, but the ionization constant of its conjugate base, NH3, is listed as 1.8 \(×\) 10−5.
\({\text{NH}}_{4}{}^{\text{+}}\) is the slightly stronger acid (Ka for \({\text{NH}}_{4}{}^{\text{+}}\) = 5.6 \(×\) 10−10).
Acid-Base Equilibrium Calculations
The chapter on chemical equilibria introduced several types of equilibrium calculations and the various mathematical strategies that are helpful in performing them. These strategies are generally useful for equilibrium systems regardless of chemical reaction class, and so they may be effectively applied to acid-base equilibrium problems. This section presents several example exercises involving equilibrium calculations for acid-base systems.

Determination of Ka from Equilibrium Concentrations Acetic acid is the principal ingredient in vinegar ((Figure)) that provides its sour taste. At equilibrium, a solution contains [CH3CO2H] = 0.0787 M and \(\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]=\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{\text{−}}\right]=0.00118\phantom{\rule{0.2em}{0ex}}M.\) What is the value of Ka for acetic acid?

Solution The relevant equilibrium equation and its equilibrium constant expression are shown below. Substitution of the provided equilibrium concentrations permits a straightforward calculation of the Ka for acetic acid.
Check Your Learning The \({\text{HSO}}_{4}{}^{\text{−}}\) ion, weak acid used in some household cleansers:
What is the acid ionization constant for this weak acid if an equilibrium mixture has the following composition: \(\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\) = 0.027 M; \(\left[{\text{HSO}}_{4}{}^{\text{−}}\right]=0.29\phantom{\rule{0.2em}{0ex}}M;\) and \(\left[{\text{SO}}_{4}{}^{2-}\right]=0.13\phantom{\rule{0.2em}{0ex}}M?\)
Ka for \({\text{HSO}}_{4}{}^{\text{−}}\) = 1.2 \(×\) 10−2
Determination of Kb from Equilibrium Concentrations Caffeine, C8H10N4O2 is a weak base. What is the value of Kb for caffeine if a solution at equilibrium has [C8H10N4O2] = 0.050 M, \(\left[{\text{C}}_{8}{\text{H}}_{10}{\text{N}}_{4}{\text{O}}_{2}{\text{H}}^{\text{+}}\right]\) = 5.0 \(×\) 10−3M, and [OH−] = 2.5 \(×\) 10−3M?
Solution The relevant equilibrium equation and its equilibrium constant expression are shown below. Substitution of the provided equilibrium concentrations permits a straightforward calculation of the Kb for caffeine.
Check Your Learning What is the equilibrium constant for the ionization of the \({\text{HPO}}_{4}{}^{2-}\) ion, a weak base
if the composition of an equilibrium mixture is as follows: [OH−] = 1.3 \(×\) 10−6M; \(\left[{\text{H}}_{2}{\text{PO}}_{4}{}^{\text{−}}\right]=0.042\phantom{\rule{0.2em}{0ex}}M;\) and \(\left[{\text{HPO}}_{4}{}^{2-}\right]=0.341\phantom{\rule{0.2em}{0ex}}M?\)
Kb for \({\text{HPO}}_{4}{}^{2-}=1.6\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-7}\)
Determination of Ka or Kb from pH The pH of a 0.0516-M solution of nitrous acid, HNO2, is 2.34. What is its Ka?
Solution The nitrous acid concentration provided is a formal concentration, one that does not account for any chemical equilibria that may be established in solution. Such concentrations are treated as “initial” values for equilibrium calculations using the ICE table approach. Notice the initial value of hydronium ion is listed as approximately zero because a small concentration of H3O+ is present (1 × 10−7M) due to the autoprotolysis of water. In many cases, such as all the ones presented in this chapter, this concentration is much less than that generated by ionization of the acid (or base) in question and may be neglected.
The pH provided is a logarithmic measure of the hydronium ion concentration resulting from the acid ionization of the nitrous acid, and so it represents an “equilibrium” value for the ICE table:
The ICE table for this system is then
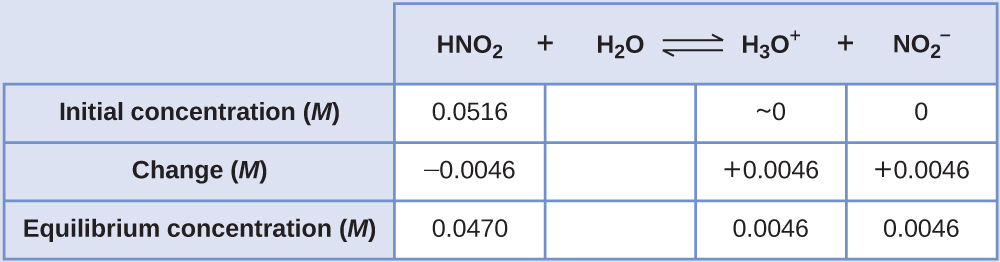
Finally, calculate the value of the equilibrium constant using the data in the table:
Check Your Learning. The pH of a solution of household ammonia, a 0.950-M solution of NH3, is 11.612. What is Kb for NH3.
Kb = 1.8 \(×\) 10−5
Calculating Equilibrium Concentrations in a Weak Acid Solution Formic acid, HCO2H, is one irritant that causes the body’s reaction to some ant bites and stings ((Figure)).

What is the concentration of hydronium ion and the pH of a 0.534-M solution of formic acid?
Solution
The ICE table for this system is
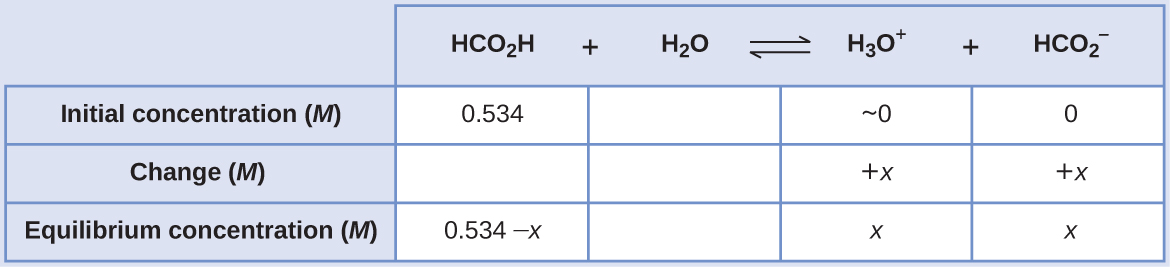
Substituting the equilibrium concentration terms into the Ka expression gives
The relatively large initial concentration and small equilibrium constant permits the simplifying assumption that x will be much lesser than 0.534, and so the equation becomes
Solving the equation for x yields
To check the assumption that x is small compared to 0.534, its relative magnitude can be estimated:
Because x is less than 5% of the initial concentration, the assumption is valid.
As defined in the ICE table, x is equal to the equilibrium concentration of hydronium ion:
Finally, the pH is calculated to be
Check Your Learning Only a small fraction of a weak acid ionizes in aqueous solution. What is the percent ionization of a 0.100-M solution of acetic acid, CH3CO2H?
percent ionization = 1.3%
Calculating Equilibrium Concentrations in a Weak Base Solution Find the concentration of hydroxide ion, the pOH, and the pH of a 0.25-M solution of trimethylamine, a weak base:
Solution The ICE table for this system is
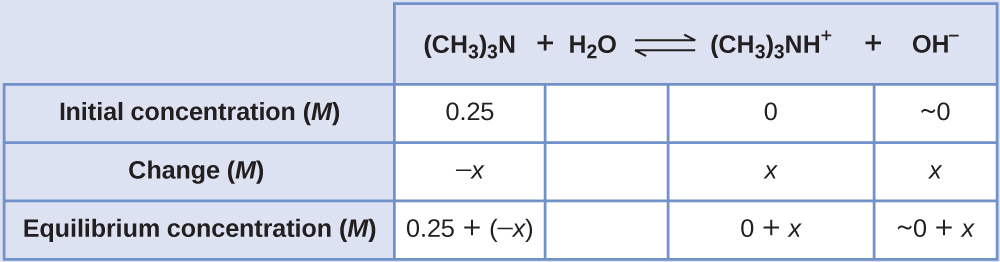
Substituting the equilibrium concentration terms into the Kb expression gives
Assuming x << 0.25 and solving for x yields
This value is less than 5% of the initial concentration (0.25), so the assumption is justified.
As defined in the ICE table, x is equal to the equilibrium concentration of hydroxide ion:
The pOH is calculated to be
Using the relation introduced in the previous section of this chapter:
permits the computation of pH:
Check Your LearningCalculate the hydroxide ion concentration and the percent ionization of a 0.0325-M solution of ammonia, a weak base with a Kb of 1.76 \(×\) 10−5.
7.56 \(×\) 10−4M, 2.33%
In some cases, the strength of the weak acid or base and its formal (initial) concentration result in an appreciable ionization. Though the ICE strategy remains effective for these systems, the algebra is a bit more involved because the simplifying assumption that x is negligible can not be made. Calculations of this sort are demonstrated in (Figure) below.
Calculating Equilibrium Concentrations without Simplifying Assumptions Sodium bisulfate, NaHSO4, is used in some household cleansers as a source of the \({\text{HSO}}_{4}{}^{\text{−}}\) ion, a weak acid. What is the pH of a 0.50-M solution of \({\text{HSO}}_{4}{}^{\text{−}}?\)
Solution The ICE table for this system is
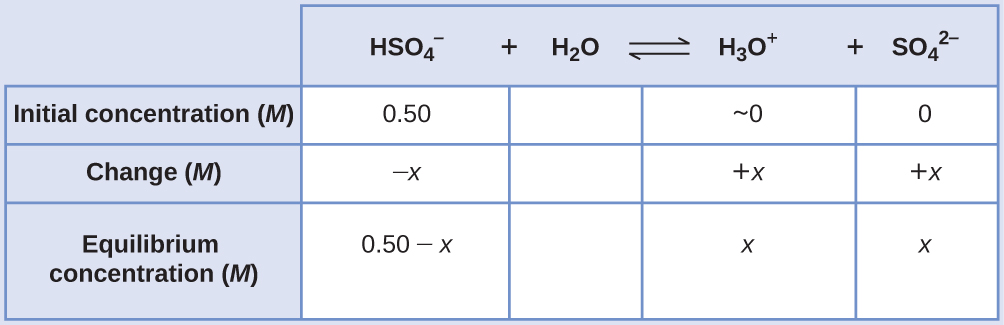
Substituting the equilibrium concentration terms into the Ka expression gives
If the assumption that x << 0.5 is made, simplifying and solving the above equation yields
This value of x is clearly not significantly less than 0.50 M; rather, it is approximately 15% of the initial concentration:
When we check the assumption, we calculate:
Because the simplifying assumption is not valid for this system, the equilibrium constant expression is solved as follows:
Rearranging this equation yields
Writing the equation in quadratic form gives
Solving for the two roots of this quadratic equation results in a negative value that may be discarded as physically irrelevant and a positive value equal to x. As defined in the ICE table, x is equal to the hydronium concentration.
Check Your Learning Calculate the pH in a 0.010-M solution of caffeine, a weak base:
pH 11.16
Effect of Molecular Structure on Acid-Base Strength
Binary Acids and Bases
In the absence of any leveling effect, the acid strength of binary compounds of hydrogen with nonmetals (A) increases as the H-A bond strength decreases down a group in the periodic table. For group 17, the order of increasing acidity is HF < HCl < HBr < HI. Likewise, for group 16, the order of increasing acid strength is H2O < H2S < H2Se < H2Te.
Across a row in the periodic table, the acid strength of binary hydrogen compounds increases with increasing electronegativity of the nonmetal atom because the polarity of the H-A bond increases. Thus, the order of increasing acidity (for removal of one proton) across the second row is CH4 < NH3 < H2O < HF; across the third row, it is SiH4 < PH3 < H2S < HCl (see (Figure)).
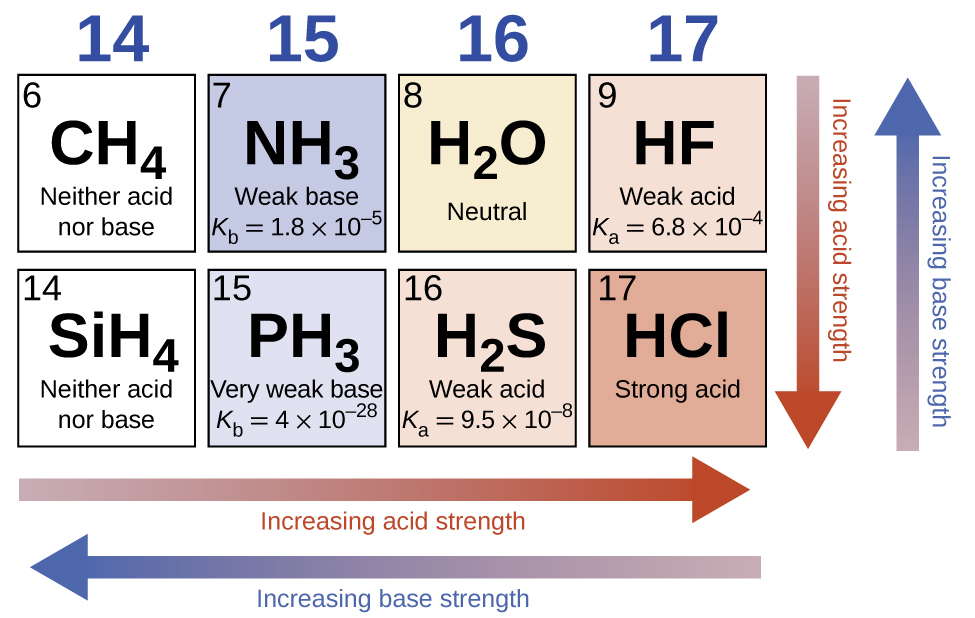
Ternary Acids and Bases
Ternary compounds composed of hydrogen, oxygen, and some third element (“E”) may be structured as depicted in the image below. In these compounds, the central E atom is bonded to one or more O atoms, and at least one of the O atoms is also bonded to an H atom, corresponding to the general molecular formula OmE(OH)n. These compounds may be acidic, basic, or amphoteric depending on the properties of the central E atom. Examples of such compounds include sulfuric acid, O2S(OH)2, sulfurous acid, OS(OH)2, nitric acid, O2NOH, perchloric acid, O3ClOH, aluminum hydroxide, Al(OH)3, calcium hydroxide, Ca(OH)2, and potassium hydroxide, KOH:
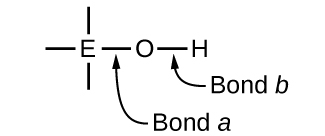
If the central atom, E, has a low electronegativity, its attraction for electrons is low. Little tendency exists for the central atom to form a strong covalent bond with the oxygen atom, and bond a between the element and oxygen is more readily broken than bond b between oxygen and hydrogen. Hence bond a is ionic, hydroxide ions are released to the solution, and the material behaves as a base—this is the case with Ca(OH)2 and KOH. Lower electronegativity is characteristic of the more metallic elements; hence, the metallic elements form ionic hydroxides that are by definition basic compounds.
If, on the other hand, the atom E has a relatively high electronegativity, it strongly attracts the electrons it shares with the oxygen atom, making bond a relatively strongly covalent. The oxygen-hydrogen bond, bond b, is thereby weakened because electrons are displaced toward E. Bond b is polar and readily releases hydrogen ions to the solution, so the material behaves as an acid. High electronegativities are characteristic of the more nonmetallic elements. Thus, nonmetallic elements form covalent compounds containing acidic −OH groups that are called oxyacids.
Increasing the oxidation number of the central atom E also increases the acidity of an oxyacid because this increases the attraction of E for the electrons it shares with oxygen and thereby weakens the O-H bond. Sulfuric acid, H2SO4, or O2S(OH)2 (with a sulfur oxidation number of +6), is more acidic than sulfurous acid, H2SO3, or OS(OH)2 (with a sulfur oxidation number of +4). Likewise nitric acid, HNO3, or O2NOH (N oxidation number = +5), is more acidic than nitrous acid, HNO2, or ONOH (N oxidation number = +3). In each of these pairs, the oxidation number of the central atom is larger for the stronger acid ((Figure)).
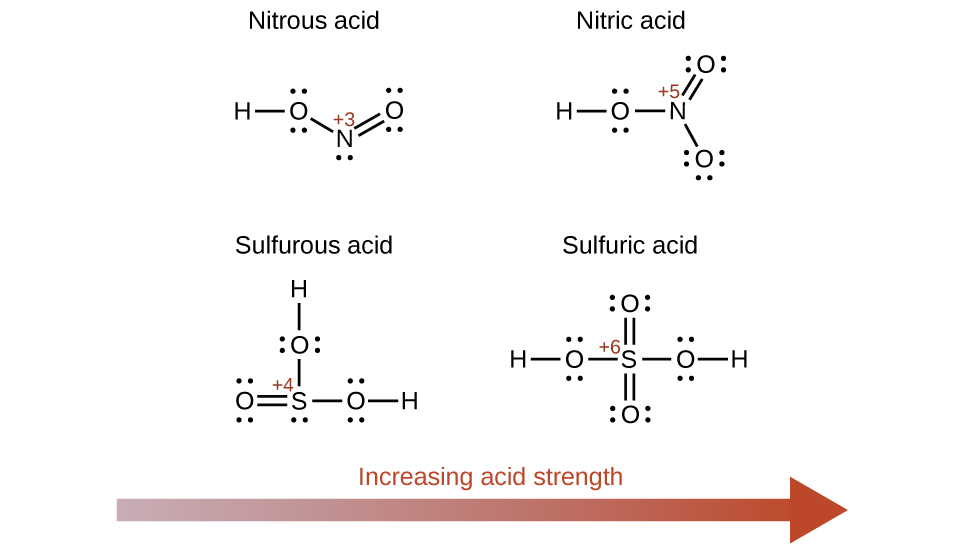
Hydroxy compounds of elements with intermediate electronegativities and relatively high oxidation numbers (for example, elements near the diagonal line separating the metals from the nonmetals in the periodic table) are usually amphoteric. This means that the hydroxy compounds act as acids when they react with strong bases and as bases when they react with strong acids. The amphoterism of aluminum hydroxide, which commonly exists as the hydrate Al(H2O)3(OH)3, is reflected in its solubility in both strong acids and strong bases. In strong bases, the relatively insoluble hydrated aluminum hydroxide, Al(H2O)3(OH)3, is converted into the soluble ion, \({\left[\text{Al}{\left({\text{H}}_{2}\text{O}\right)}_{2}\left({\text{OH}\right)}_{4}\right]}^{\text{−}},\) by reaction with hydroxide ion:
In this reaction, a proton is transferred from one of the aluminum-bound H2O molecules to a hydroxide ion in solution. The Al(H2O)3(OH)3 compound thus acts as an acid under these conditions. On the other hand, when dissolved in strong acids, it is converted to the soluble ion \({\left[\text{Al}{\left({\text{H}}_{2}\text{O}\right)}_{6}\right]}^{3+}\) by reaction with hydronium ion:
In this case, protons are transferred from hydronium ions in solution to Al(H2O)3(OH)3, and the compound functions as a base.
Key Concepts and Summary
The relative strengths of acids and bases are reflected in the magnitudes of their ionization constants; the stronger the acid or base, the larger its ionization constant. A reciprocal relation exists between the strengths of a conjugate acid-base pair: the stronger the acid, the weaker its conjugate base. Water exerts a leveling effect on dissolved acids or bases, reacting completely to generate its characteristic hydronium and hydroxide ions (the strongest acid and base that may exist in water). The strengths of the binary acids increase from left to right across a period of the periodic table (CH4 < NH3 < H2O < HF), and they increase down a group (HF < HCl < HBr < HI). The strengths of oxyacids that contain the same central element increase as the oxidation number of the element increases (H2SO3 < H2SO4). The strengths of oxyacids also increase as the electronegativity of the central element increases [H2SeO4 < H2SO4].
Key Equations
- \({K}_{\text{a}}=\phantom{\rule{0.2em}{0ex}}\frac{\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\left[{\text{A}}^{\text{−}}\right]}{\left[\text{HA}\right]}\)
- \({K}_{\text{b}}=\phantom{\rule{0.2em}{0ex}}\frac{\left[{\text{HB}}^{\text{+}}\right]\left[{\text{OH}}^{\text{−}}\right]}{\left[\text{B}\right]}\)
- Ka\(×\)Kb = 1.0 \(×\) 10−14 = Kw
- \(\text{Percent ionization}=\phantom{\rule{0.2em}{0ex}}\frac{{\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]}_{\text{eq}}}{{\left[\text{HA]}}_{0}}\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}100\)
Chemistry End of Chapter Exercises
Explain why the neutralization reaction of a strong acid and a weak base gives a weakly acidic solution.
Explain why the neutralization reaction of a weak acid and a strong base gives a weakly basic solution.
The salt ionizes in solution, but the anion slightly reacts with water to form the weak acid. This reaction also forms OH−, which causes the solution to be basic.
Use this list of important industrial compounds (and (Figure)) to answer the following questions regarding: CaO, Ca(OH)2, CH3CO2H, CO2, HCl, H2CO3, HF, HNO2, HNO3, H3PO4, H2SO4, NH3, NaOH, Na2CO3.
(a) Identify the strong Brønsted-Lowry acids and strong Brønsted-Lowry bases.
(b) List those compounds in (a) that can behave as Brønsted-Lowry acids with strengths lying between those of H3O+ and H2O.
(c) List those compounds in (a) that can behave as Brønsted-Lowry bases with strengths lying between those of H2O and OH−.
The odor of vinegar is due to the presence of acetic acid, CH3CO2H, a weak acid. List, in order of descending concentration, all of the ionic and molecular species present in a 1-M aqueous solution of this acid.
[H2O] > [CH3CO2H] > \(\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\) ≈ \(\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{\text{−}}\right]\) > [OH−]
Household ammonia is a solution of the weak base NH3 in water. List, in order of descending concentration, all of the ionic and molecular species present in a 1-M aqueous solution of this base.
Explain why the ionization constant, Ka, for H2SO4 is larger than the ionization constant for H2SO3.
The oxidation state of the sulfur in H2SO4 is greater than the oxidation state of the sulfur in H2SO3.
Explain why the ionization constant, Ka, for HI is larger than the ionization constant for HF.
Gastric juice, the digestive fluid produced in the stomach, contains hydrochloric acid, HCl. Milk of Magnesia, a suspension of solid Mg(OH)2 in an aqueous medium, is sometimes used to neutralize excess stomach acid. Write a complete balanced equation for the neutralization reaction, and identify the conjugate acid-base pairs.
\(\begin{array}{cccccc}\text{Mg}{\left(\text{OH}\right)}_{2}\left(s\right)+& \text{2HCl}\left(aq\right)& \phantom{\rule{0.2em}{0ex}}⟶\phantom{\rule{0.2em}{0ex}}& {\text{Mg}}^{2+}\left(aq\right)+& 2{\text{Cl}}^{\text{−}}\left(aq\right)+\phantom{\rule{0.2em}{0ex}}& {\text{2H}}_{2}\text{O}\left(l\right)\\ \text{BB}& \text{BA}& & \text{CB}& \text{CA}& \end{array}\)
Nitric acid reacts with insoluble copper(II) oxide to form soluble copper(II) nitrate, Cu(NO3)2, a compound that has been used to prevent the growth of algae in swimming pools. Write the balanced chemical equation for the reaction of an aqueous solution of HNO3 with CuO.
What is the ionization constant at 25 °C for the weak acid \({\text{CH}}_{3}{\text{NH}}_{3}{}^{\text{+}},\) the conjugate acid of the weak base CH3NH2, Kb = 4.4 \(×\) 10−4.
\({K}_{\text{a}}=2.3\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-11}\)
What is the ionization constant at 25 °C for the weak acid \({\left({\text{CH}}_{3}\right)}_{2}{\text{NH}}_{2}{}^{\text{+}},\) the conjugate acid of the weak base (CH3)2NH, Kb = 5.9 \(×\) 10−4?
Which base, CH3NH2 or (CH3)2NH, is the stronger base? Which conjugate acid, \({\left({\text{CH}}_{3}\right)}_{2}{\text{NH}}_{2}{}^{\text{+}}\) or \({\text{CH}}_{3}{\text{NH}}_{3}{}^{\text{+}}\), is the stronger acid?
The stronger base or stronger acid is the one with the larger Kb or Ka, respectively. In these two examples, they are (CH3)2NH and \({\text{CH}}_{3}{\text{NH}}_{3}{}^{\text{+}}.\)
Which is the stronger acid, \({\text{NH}}_{4}{}^{\text{+}}\) or HBrO?
Which is the stronger base, (CH3)3N or \({\text{H}}_{2}{\text{BO}}_{3}{}^{\text{−}}?\)
triethylamine
Predict which acid in each of the following pairs is the stronger and explain your reasoning for each.
(a) H2O or HF
(b) B(OH)3 or Al(OH)3
(c) \({\text{HSO}}_{3}{}^{\text{−}}\) or \({\text{HSO}}_{4}{}^{\text{−}}\)
(d) NH3 or H2S
(e) H2O or H2Te
Predict which compound in each of the following pairs of compounds is more acidic and explain your reasoning for each.
(a) \({\text{HSO}}_{4}{}^{\text{−}}\) or \({\text{HSeO}}_{4}{}^{\text{−}}\)
(b) NH3 or H2O
(c) PH3 or HI
(d) NH3 or PH3
(e) H2S or HBr
(a) \({\text{HSO}}_{4}{}^{\text{−}};\) higher electronegativity of the central ion. (b) H2O; NH3 is a base and water is neutral, or decide on the basis of Ka values. (c) HI; PH3 is weaker than HCl; HCl is weaker than HI. Thus, PH3 is weaker than HI. (d) PH3; in binary compounds of hydrogen with nonmetals, the acidity increases for the element lower in a group. (e) HBr; in a period, the acidity increases from left to right; in a group, it increases from top to bottom. Br is to the left and below S, so HBr is the stronger acid.
Rank the compounds in each of the following groups in order of increasing acidity or basicity, as indicated, and explain the order you assign.
(a) acidity: HCl, HBr, HI
(b) basicity: H2O, OH−, H−, Cl−
(c) basicity: Mg(OH)2, Si(OH)4, ClO3(OH) (Hint: Formula could also be written as HClO4.)
(d) acidity: HF, H2O, NH3, CH4
Rank the compounds in each of the following groups in order of increasing acidity or basicity, as indicated, and explain the order you assign.
(a) acidity: NaHSO3, NaHSeO3, NaHSO4
(b) basicity: \({\text{BrO}}_{2}{}^{\text{−}},\) \({\text{ClO}}_{2}{}^{\text{−}},\) \({\text{IO}}_{2}{}^{\text{−}}\)
(c) acidity: HOCl, HOBr, HOI
(d) acidity: HOCl, HOClO, HOClO2, HOClO3
(e) basicity: \({\text{NH}}_{2}{}^{\text{−}},\) HS−, HTe−, \({\text{PH}}_{2}{}^{\text{−}}\)
(f) basicity: BrO−, \({\text{BrO}}_{2}{}^{\text{−}},\) \({\text{BrO}}_{3}{}^{\text{−}},\) \({\text{BrO}}_{4}{}^{\text{−}}\)
(a) NaHSeO3 < NaHSO3 < NaHSO4; in polyoxy acids, the more electronegative central element—S, in this case—forms the stronger acid. The larger number of oxygen atoms on the central atom (giving it a higher oxidation state) also creates a greater release of hydrogen atoms, resulting in a stronger acid. As a salt, the acidity increases in the same manner. (b) \({\text{ClO}}_{2}{}^{\text{−}}<{\text{BrO}}_{2}{}^{\text{−}}<{\text{IO}}_{2}{}^{\text{−}};\) the basicity of the anions in a series of acids will be the opposite of the acidity in their oxyacids. The acidity increases as the electronegativity of the central atom increases. Cl is more electronegative than Br, and I is the least electronegative of the three. (c) HOI < HOBr < HOCl; in a series of the same form of oxyacids, the acidity increases as the electronegativity of the central atom increases. Cl is more electronegative than Br, and I is the least electronegative of the three. (d) HOCl < HOClO < HOClO2 < HOClO3; in a series of oxyacids of the same central element, the acidity increases as the number of oxygen atoms increases (or as the oxidation state of the central atom increases). (e) \({\text{HTe}}^{\text{−}}<{\text{HS}}^{\text{−}}<<\phantom{\rule{0.2em}{0ex}}{\text{PH}}_{2}{}^{\text{−}}<{\text{NH}}_{2}{}^{\text{−}};\) \({\text{PH}}_{2}{}^{\text{−}}\) and \({\text{NH}}_{2}{}^{\text{−}}\) are anions of weak bases, so they act as strong bases toward H+. \({\text{HTe}}^{\text{−}}\) and HS− are anions of weak acids, so they have less basic character. In a periodic group, the more electronegative element has the more basic anion. (f) \({\text{BrO}}_{4}{}^{\text{−}}<{\text{BrO}}_{3}{}^{\text{−}}<{\text{BrO}}_{2}{}^{\text{−}}<{\text{BrO}}^{\text{−}};\) with a larger number of oxygen atoms (that is, as the oxidation state of the central ion increases), the corresponding acid becomes more acidic and the anion consequently less basic.
Both HF and HCN ionize in water to a limited extent. Which of the conjugate bases, F− or CN−, is the stronger base?
The active ingredient formed by aspirin in the body is salicylic acid, C6H4OH(CO2H). The carboxyl group (−CO2H) acts as a weak acid. The phenol group (an OH group bonded to an aromatic ring) also acts as an acid but a much weaker acid. List, in order of descending concentration, all of the ionic and molecular species present in a 0.001-M aqueous solution of C6H4OH(CO2H).
\(\left[{\text{H}}_{2}\text{O}\right]\phantom{\rule{0.2em}{0ex}}>\left[{\text{C}}_{6}{\text{H}}_{4}\text{OH}\left({\text{CO}}_{2}\text{H}\right)\right]\phantom{\rule{0.2em}{0ex}}>{\text{[H}}^{\text{+}}\text{]}\phantom{\rule{0.2em}{0ex}}\text{0}>{\text{[C}}_{6}{\text{H}}_{4}\text{OH}{\left({\text{CO}}_{2}\right)}^{\text{−}}\right]\gg \left[{\text{C}}_{6}{\text{H}}_{4}\text{O}{\left({\text{CO}}_{2}\text{H}\right)}^{\text{−}}\right]\phantom{\rule{0.2em}{0ex}}>\left[{\text{OH}}^{\text{−}}\right]\)
Are the concentrations of hydronium ion and hydroxide ion in a solution of an acid or a base in water directly proportional or inversely proportional? Explain your answer.
What two common assumptions can simplify calculation of equilibrium concentrations in a solution of a weak acid or base?
1. Assume that the change in initial concentration of the acid as the equilibrium is established can be neglected, so this concentration can be assumed constant and equal to the initial value of the total acid concentration. 2. Assume we can neglect the contribution of water to the equilibrium concentration of H3O+.
Which of the following will increase the percent of NH3 that is converted to the ammonium ion in water?
(a) addition of NaOH
(b) addition of HCl
(c) addition of NH4Cl
(b) The addition of HCl
Which of the following will increase the percentage of HF that is converted to the fluoride ion in water?
(a) addition of NaOH
(b) addition of HCl
(c) addition of NaF
What is the effect on the concentrations of \({\text{NO}}_{2}{}^{\text{−}},\) HNO2, and OH− when the following are added to a solution of KNO2 in water:
(a) HCl
(b) HNO2
(c) NaOH
(d) NaCl
(e) KNO
(a) Adding HCl will add H3O+ ions, which will then react with the OH− ions, lowering their concentration. The equilibrium will shift to the right, increasing the concentration of HNO2, and decreasing the concentration of \({\text{NO}}_{2}{}^{\text{−}}\) ions. (b) Adding HNO2 increases the concentration of HNO2 and shifts the equilibrium to the left, increasing the concentration of \({\text{NO}}_{2}{}^{\text{−}}\) ions and decreasing the concentration of OH− ions. (c) Adding NaOH adds OH− ions, which shifts the equilibrium to the left, increasing the concentration of \({\text{NO}}_{2}{}^{\text{−}}\) ions and decreasing the concentrations of HNO2. (d) Adding NaCl has no effect on the concentrations of the ions. (e) Adding KNO2 adds \({\text{NO}}_{2}{}^{\text{−}}\) ions and shifts the equilibrium to the right, increasing the HNO2 and OH− ion concentrations.
What is the effect on the concentration of hydrofluoric acid, hydronium ion, and fluoride ion when the following are added to separate solutions of hydrofluoric acid?
(a) HCl
(b) KF
(c) NaCl
(d) KOH
(e) HF
Why is the hydronium ion concentration in a solution that is 0.10 M in HCl and 0.10 M in HCOOH determined by the concentration of HCl?
This is a case in which the solution contains a mixture of acids of different ionization strengths. In solution, the HCO2H exists primarily as HCO2H molecules because the ionization of the weak acid is suppressed by the strong acid. Therefore, the HCO2H contributes a negligible amount of hydronium ions to the solution. The stronger acid, HCl, is the dominant producer of hydronium ions because it is completely ionized. In such a solution, the stronger acid determines the concentration of hydronium ions, and the ionization of the weaker acid is fixed by the [H3O+] produced by the stronger acid.
From the equilibrium concentrations given, calculate Ka for each of the weak acids and Kb for each of the weak bases.
(a) CH3CO2H: \(\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\) = 1.34 \(×\) 10−3M;
\(\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{\text{−}}\right]\) = 1.34 \(×\) 10−3M;
[CH3CO2H] = 9.866 \(×\) 10−2M;
(b) ClO−: [OH−] = 4.0 \(×\) 10−4M;
[HClO] = 2.38 \(×\) 10−4M;
[ClO−] = 0.273 M;
(c) HCO2H: [HCO2H] = 0.524 M;
\(\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\) = 9.8 \(×\) 10−3M;
\(\left[{\text{HCO}}_{2}{}^{\text{−}}\right]\) = 9.8 \(×\) 10−3M;
(d) \({\text{C}}_{6}{\text{H}}_{5}{\text{NH}}_{3}{}^{\text{+}}:\) \(\left[{\text{C}}_{6}{\text{H}}_{5}{\text{NH}}_{3}{}^{\text{+}}\right]\) = 0.233 M;
[C6H5NH2] = 2.3 \(×\) 10−3M;
\(\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\) = 2.3 \(×\) 10−3M
From the equilibrium concentrations given, calculate Ka for each of the weak acids and Kb for each of the weak bases.
(a) NH3: [OH−] = 3.1 \(×\) 10−3M;
\(\left[{\text{NH}}_{4}{}^{\text{+}}\right]\) = 3.1 \(×\) 10−3M;
[NH3] = 0.533 M;
(b) HNO2: \(\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\) = 0.011 M;
\(\left[{\text{NO}}_{2}{}^{\text{−}}\right]\) = 0.0438 M;
[HNO2] = 1.07 M;
(c) (CH3)3N: [(CH3)3N] = 0.25 M;
[(CH3)3NH+] = 4.3 \(×\) 10−3M;
[OH−] = 3.7 \(×\) 10−3M;
(d) \({\text{NH}}_{4}{}^{\text{+}}:\) \(\left[{\text{NH}}_{4}{}^{\text{+}}\right]\) = 0.100 M;
[NH3] = 7.5 \(×\) 10−6M;
[H3O+] = 7.5 \(×\) 10−6M
(a) \({K}_{\text{b}}=1.8\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-5};\) (b) \({K}_{\text{a}}=4.5\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-4};\) (c) \({K}_{\text{b}}=7.4\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-5};\) (d) \({K}_{\text{a}}=5.6\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-10}\)
Determine Kb for the nitrite ion, \({\text{NO}}_{2}{}^{\text{−}}.\) In a 0.10-M solution this base is 0.0015% ionized.
Determine Ka for hydrogen sulfate ion, \({\text{HSO}}_{4}{}^{\text{−}}.\) In a 0.10-M solution the acid is 29% ionized.
\({K}_{\text{a}}=1.2\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-2}\)
Calculate the ionization constant for each of the following acids or bases from the ionization constant of its conjugate base or conjugate acid:
(a) F−
(b) \({\text{NH}}_{4}{}^{\text{+}}\)
(c) \({\text{AsO}}_{4}{}^{3-}\)
(d) \({\left({\text{CH}}_{3}\right)}_{2}{\text{NH}}_{2}{}^{\text{+}}\)
(e) \({\text{NO}}_{2}{}^{\text{−}}\)
(f) \({\text{HC}}_{2}{\text{O}}_{4}{}^{\text{−}}\) (as a base)
Calculate the ionization constant for each of the following acids or bases from the ionization constant of its conjugate base or conjugate acid:
(a) HTe− (as a base)
(b) \({\left({\text{CH}}_{3}\right)}_{3}{\text{NH}}^{\text{+}}\)
(c) \({\text{HAsO}}_{4}{}^{3-}\) (as a base)
(d) \({\text{HO}}_{2}{}^{\text{−}}\) (as a base)
(e) \({\text{C}}_{6}{\text{H}}_{5}{\text{NH}}_{3}{}^{\text{+}}\)
(f) \({\text{HSO}}_{3}{}^{\text{−}}\) (as a base)
(a) \({K}_{\text{b}}=4.3\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-12}\) (b) \({K}_{\text{a}}=1.6\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-8}\) (c) \({K}_{\text{b}}=5.9\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-7}\) (d) \({K}_{\text{b}}=4.2\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-3}\) (e) \({K}_{\text{b}}=2.3\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-3}\) (f) \({K}_{\text{b}}=6.3\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-13}\)
Using the Ka value of 1.4 \(×\) 10−5, place \(\text{Al}{\left({\text{H}}_{2}\text{O}\right)}_{6}{}^{3+}\) in the correct location in (Figure).
Calculate the concentration of all solute species in each of the following solutions of acids or bases. Assume that the ionization of water can be neglected, and show that the change in the initial concentrations can be neglected.
(a) 0.0092 M HClO, a weak acid
(b) 0.0784 M C6H5NH2, a weak base
(c) 0.0810 M HCN, a weak acid
(d) 0.11 M (CH3)3N, a weak base
(e) 0.120 M \(\text{Fe}{\left({\text{H}}_{2}\text{O}\right)}_{6}{}^{2+}\) a weak acid, Ka = 1.6 \(×\) 10−7
(a) \(\frac{\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\left[{\text{ClO}}^{\text{−}}\right]}{\left[\text{HClO}\right]}\phantom{\rule{0.2em}{0ex}}=\phantom{\rule{0.2em}{0ex}}\frac{\left(x\right)\left(x\right)}{\left(0.0092-x\right)}\phantom{\rule{0.2em}{0ex}}\approx \phantom{\rule{0.2em}{0ex}}\frac{\left(x\right)\left(x\right)}{0.0092}\phantom{\rule{0.2em}{0ex}}=2.9\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-8}\)
Solving for x gives 1.63 \(×\) 10−5M. This value is less than 5% of 0.0092, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are:
[H3O+] = [ClO] = 5.8 \(×\) 10−5M
[HClO] = 0.00092 M
[OH−] = 6.1 \(×\) 10−10M;
(b) \(\frac{\left[{\text{C}}_{6}{\text{H}}_{5}{\text{NH}}_{3}{}^{\text{+}}\right]\left[{\text{OH}}^{\text{−}}\right]}{\left[{\text{C}}_{6}{\text{H}}_{5}{\text{NH}}_{2}\right]}\phantom{\rule{0.2em}{0ex}}=\phantom{\rule{0.2em}{0ex}}\frac{\left(x\right)\left(x\right)}{\left(0.0784-x\right)}\phantom{\rule{0.2em}{0ex}}\approx \phantom{\rule{0.2em}{0ex}}\frac{\left(x\right)\left(x\right)}{0.0784}\phantom{\rule{0.2em}{0ex}}=4.3\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-10}\)
Solving for x gives 5.81 \(×\) 10−6M. This value is less than 5% of 0.0784, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are:
\(\left[{\text{CH}}_{3}{\text{CO}}_{2}{}^{\text{−}}\right]\) = [OH−] = 5.8 \(×\) 10−6M
[C6H5NH2] = 0.00784
[H3O+] = 1.7\(×\) 10−9M;
(c) \(\frac{\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]\left[{\text{CN}}^{\text{−}}\right]}{\left[\text{HCN}\right]}\phantom{\rule{0.2em}{0ex}}=\phantom{\rule{0.2em}{0ex}}\frac{\left(x\right)\left(x\right)}{\left(0.0810-x\right)}\phantom{\rule{0.2em}{0ex}}\approx \phantom{\rule{0.2em}{0ex}}\frac{\left(x\right)\left(x\right)}{0.0810}\phantom{\rule{0.2em}{0ex}}=4.9\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-10}\)
Solving for x gives 6.30 \(×\) 10−6M. This value is less than 5% of 0.0810, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are:
[H3O+] = [CN−] = 6.3 \(×\) 10−6M
[HCN] = 0.0810 M
[OH−] = 1.6 \(×\) 10−9M;
(d) \(\frac{\left[{\left({\text{CH}}_{3}\right)}_{3}{\text{NH}}^{\text{+}}\right]\left[{\text{OH}}^{\text{−}}\right]}{\left[{\left({\text{CH}}_{3}\right)}_{3}\text{N}\right]}\phantom{\rule{0.2em}{0ex}}=\phantom{\rule{0.2em}{0ex}}\frac{\left(x\right)\left(x\right)}{\left(0.11-x\right)}\phantom{\rule{0.2em}{0ex}}\approx \phantom{\rule{0.2em}{0ex}}\frac{\left(x\right)\left(x\right)}{0.11}\phantom{\rule{0.2em}{0ex}}=6.3\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-5}\)
Solving for x gives 2.63 \(×\) 10−3M. This value is less than 5% of 0.11, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are:
[(CH3)3NH+] = [OH−] = 2.6 \(×\) 10−3M
[(CH3)3N] = 0.11 M
[H3O+] = 3.8 \(×\) 10−12M;
(e) \(\frac{\left[\text{Fe}{\left({\text{H}}_{2}\text{O}\right)}_{5}\left({\text{OH}\right)}^{\text{+}}\right]\left[{\text{H}}_{3}{\text{O}}^{\text{+}}\right]}{\left[\text{Fe}{\left({\text{H}}_{2}\text{O}\right)}_{6}{}^{2+}\right]}\phantom{\rule{0.2em}{0ex}}=\phantom{\rule{0.2em}{0ex}}\frac{\left(x\right)\left(x\right)}{\left(0.120-x\right)}\phantom{\rule{0.2em}{0ex}}\approx \phantom{\rule{0.2em}{0ex}}\frac{\left(x\right)\left(x\right)}{0.120}\phantom{\rule{0.2em}{0ex}}=1.6\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-7}\)
Solving for x gives 1.39 \(×\) 10−4M. This value is less than 5% of 0.120, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are:
[Fe(H2O)5(OH)+] = [H3O+] = 1.4 \(×\) 10−4M
\(\left[\text{Fe}{\left({\text{H}}_{2}\text{O}\right)}_{6}{}^{2+}\right]\) = 0.120 M
[OH−] = 7.2 \(×\) 10−11M
Propionic acid, C2H5CO2H (Ka = 1.34 \(×\) 10−5), is used in the manufacture of calcium propionate, a food preservative. What is the pH of a 0.698-M solution of C2H5CO2H?
White vinegar is a 5.0% by mass solution of acetic acid in water. If the density of white vinegar is 1.007 g/cm3, what is the pH?
pH = 2.41
The ionization constant of lactic acid, CH3CH(OH)CO2H, an acid found in the blood after strenuous exercise, is 1.36 \(×\) 10−4. If 20.0 g of lactic acid is used to make a solution with a volume of 1.00 L, what is the concentration of hydronium ion in the solution?
Nicotine, C10H14N2, is a base that will accept two protons (Kb1 = 7 \(×\) 10−7, Kb2 = 1.4 \(×\) 10−11). What is the concentration of each species present in a 0.050-M solution of nicotine?
[C10H14N2] = 0.049 M; [C10H14N2H+] = 1.9 \(×\) 10−4M; \(\left[{\text{C}}_{10}{\text{H}}_{14}{\text{N}}_{2}{\text{H}}_{2}{}^{2+}\right]\) = 1.4 \(×\) 10−11M; [OH−] = 1.9 \(×\) 10−4M; [H3O+] = 5.3 \(×\) 10−11M
The pH of a 0.23-M solution of HF is 1.92. Determine Ka for HF from these data.
The pH of a 0.15-M solution of \({\text{HSO}}_{4}{}^{\text{−}}\) is 1.43. Determine Ka for \({\text{HSO}}_{4}{}^{\text{−}}\) from these data.
\({K}_{\text{a}}=1.2\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-2}\)
The pH of a 0.10-M solution of caffeine is 11.70. Determine Kb for caffeine from these data:
\({\text{C}}_{8}{\text{H}}_{10}{\text{N}}_{4}{\text{O}}_{2}\left(aq\right)+{\text{H}}_{2}\text{O}\left(l\right)\phantom{\rule{0.2em}{0ex}}⇌\phantom{\rule{0.2em}{0ex}}{\text{C}}_{8}{\text{H}}_{10}{\text{N}}_{4}{\text{O}}_{2}{\text{H}}^{\text{+}}\left(aq\right)+{\text{OH}}^{\text{−}}\left(aq\right)\)
The pH of a solution of household ammonia, a 0.950 M solution of NH3, is 11.612. Determine Kb for NH3 from these data.
\({K}_{\text{b}}=1.77\phantom{\rule{0.2em}{0ex}}×\phantom{\rule{0.2em}{0ex}}{10}^{-5}\)
Glossary
- acid ionization constant (Ka)
- equilibrium constant for an acid ionization reaction
- base ionization constant (Kb)
- equilibrium constant for a base ionization reaction
- leveling effect
- observation that acid-base strength of solutes in a given solvent is limited to that of the solvent’s characteristic acid and base species (in water, hydronium and hydroxide ions, respectively)
- oxyacid
- ternary compound with acidic properties, molecules of which contain a central nonmetallic atom bonded to one or more O atoms, at least one of which is bonded to an ionizable H atom
- percent ionization
- ratio of the concentration of ionized acid to initial acid concentration expressed as a percentage

