Manual White Blood Cell Count Procedure
Manual White Blood Cell Count Procedure Using A Cyto C-Chip Disposable Hemacytometer
Learning Objectives
- Provides proper instructions for performing a manual white blood cell using a hemacytometer.
- Calculate a white blood cell count using the proper formula.
- Identify the sources of error.
- Interpret the results
CSMLS Competency Profile References
- Category 2: Data specimen collection and handling – 2.01; 2.07-2.08; 2.11; 2.12
- Category 3: Analytical processes – 3.01; 3.09.01 – 3.09.02; 3.13
- Category 4: Interpretation and reporting results – 4.01-4.06
- Category 5: Quality management – 5.02; 5.05; 5.07
- Category 6: Critical thinking – 6.04-6.06
- Safety, quality management, communication and professional practice apply to both the MLT and MLA and are categories in both competency profiles.
Principle
Capillary or venous blood is added to a diluent (2% filtered acetic acid) at a specific volume. The acetic acid will lyse the red cell leaving the WBC’s and the nucleated red blood cells (NRBC’s) intact. The nuclei of the WBC’s will be stained by the addition of methylene blue powder to the diluent. Both sides of the hemacytometer are filled with diluted blood. Allow the cells to settle for 2 minutes before performing the count.
Materials
- Filtered 2% acetic acid with new methylene blue stain (Refer to appendix 4 for preparation)
- Neubauer Hemacytometer with cover slip. (Refer to appendix #1 for use)
- Light microscope
- Micro-hematocrit capillary tubes
- Petri plate with moist gauze
- Cell counters
- 12 x 75 glass tubes
- Gauze/Kim wipes
- Eppendorf pipettes
- Timers
Sample Type
- Whole blood collected in an EDTA anticoagulated tube.
Safety Considerations
- The 2% acetic acid is considered TOXIC AND CORROSIVE!
- Refer to MSD sheets for safety considerations, located in the reagent prep room.
- All specimens received in the lab must be regarded as potentially infectious so follow safe practices.
- Refer to regulations and safety precautions in the student lab manual located in each student lab.
Procedure
- Prepare the hemocytometer for counting. Refer to appendix 1.
- Gently mix the sample before using.
- Label a 12×75 test tube with patients’ full name and MRN number.
- Pipette 1.0 mL (1000uL) of filtered 2% acetic acid into a 12×75 tube labelled tube.
- Pipette 50uL of the patients’ sample into the filtered 2% acetic acid. (Dilution factor of 20)
- Cap the tube or seal it with parafilm and mix the dilution. Let stand for a minimum of 2 minutes to allow the RBCs to lyse. Make sure you mix it again just before using.
- Pipette 10uL of sample into side A and B of the Hemocytometer chambers.
- Do not over-fill the chamber. Be careful not to introduce air bubbles when charging the chamber.
- Allow the charged hemocytometer to sit for about 2 minutes before counting. This is important to allow the cells to settle out on the hemocytometer.
- When ready to count, place the hemacytometer on the microscope stage. Note: You may have to adjust the lighting to clearly see the grid/cells.
- Using the 10X objective, focus and count all the white blood cells in the four large corner squares on both sides of the Hemacytometer.
- Calculate the total WBC count using the formula in the Interpretation section.
- Document the results on the worksheet.
Procedural Note
- Although the acetic acid is in a dilute form, avoid breathing in any fumes.
- Always keep the acetic acid covered when not using it.
- Ensure that the hemacytometer is free of dust and dirt before using.
- If there is going to be a delay in counting you need to keep the chamber in a moist environment to avoid drying out. You can moisten gauze in a petri dish and keep the hemacytometer there until you are ready to count the cells.
Quality Control
- Both sides of the Hemocytometer must agree within 10% of the mean. If they do not agree then you must repeat the test.
- Only count the cells that touch the top and left side of each large square, but do not count the cells that are touching the right side and bottom of each square. This maintains consistency in counting and more accurate results.
- Expected values (there are age dependent ranges that may vary). 3.6 – 10.6 x 109/L
INTERPRETATION
- Formula
- N = the number of WBC counted
- Average of both sides of the Hemacytometer
- D = Dilution factor
- Based on this procedure, 1.0mL of acetic acid added to 50uL of sample = 1:21.
- V = Volume (area x depth)
Example
- 4 WBC squares are 1 mm2 x 0.1mm = 0.4 mm2,
- so when you add 1.0 ml of 2 % Acetic Acid and 50 μL blood/fluid,
- you have a dilution of 1:21.
- The volume is always going to be 0.4mm3
Patient
An average of 25 cells are counted on a hemacytometer using a dilution factor of 21.
Conversion:
- The formula for calculating a manual WBC will yield a result (x 106/L). WBC counts from a blood sample are reported in x 109/L; therefore, it will have to be converted, divide the result by 1000.
- A manual WBC count may be required if the WBC estimate does not agree within 10% of the analyzer result.
- This could be caused by the presence of NRBC’s, giant platelets, platelet clumps or cryoglobulins.
NOTE
- Manual WBC counts are still the standard for counting WBC on
- CSFs.Uncommon to perform manual WBC on EDTA.
- Refer to measurements of a hemacytometer section.
Sources of Error
- Dust/dirt or air bubbles in the counting chamber can lead to erroneous results.
- Contaminated diluting fluid may cause erroneous results.
- A high WBC count may make it difficult to obtain a reasonable count, so a secondary dilution may be required.
- NRBC’s will not lyse and will interfere with the manual WBC.
- Not allowing the red cells to lyse completely may cause difficulty when counting the WBC.
- WBC may clump if the dilution is not mixed immediately after preparation, resulting in erroneous results.
- The presence of clots in the tube or fibrin strands on a peripheral blood smear is an indication of a clotted sample and must be recollected.
- Not maintaining a consistent counting routine may lead to erroneous results.
- Not allowing the chamber to settle before counting will result in erroneous results.
How to Use a Hemacytometer
Preparing the Hemocytometer.
- Remove the disposable hemocytometer from the package.
- Inspect it to make sure there are no cracks or dirt, etc.
- Label the Hemocytometer with patient name (last name, first initial) and the MRN number.
- Fill both sides of the Hemocytometer with the specimen/dilution.
- The entire surface of the chamber is counted for CSF specimens (all nine squares).
- Once the count is completed, discard the hemocytometer in the biohazard waste container.
Hemocytometer Measurements

- With CSF all nine squares are counted.
- For other fluids and EDTA specimens: White blood cells are counted in the four corner squares marked with a “W,” using a 10x low power objective.
- Platelets, not shown here, are counted in the entire center square (all twenty-five small squares), using a 40x dry objective. Not practice anymore.
- RBC counts are no longer performed manually.
Solid Circles are Counted, and the Open Circles are Not Counted
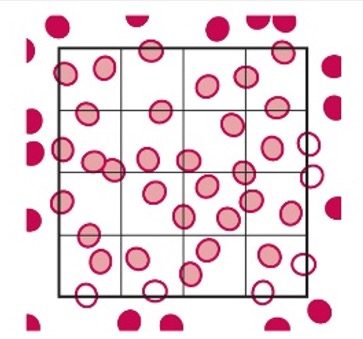
- This is a common counting strategy used in many labs to ensure accuracy in counting WBCs Count cells touching the top and left, but not the ones touching the right or bottom.
- Note: This only applies to the outside borders of the nine squares, not all squares.
Procedure for Preparation of 2% Acetic Acid with Methylene Blue
- Pour 490 mL of the Distilled Water into a 500mL cylinder.
- Carefully add 10mL of glacial acetic acid to the distilled water, bringin the total volume to 500mL.
- Cover with the parafilm and mix thoroughly.
- Add a very small amount of New Methylene blue powder to color the solution a very pale blue. The tip of a wooden applicator stick is suffient.
- Label a glass flask/bottle with appropriate safety information
- Filter the solution into a labelled glass container.
- Refrigerate the reagent until required.
