Examination of a Peripheral Blood Smear
Learning Objectives
- Prepare blood specimens for microscopic evaluation by applying the principles of bright field microscopy and
physical/chemical principles of staining - Perform staining using a Wright stain technique.
- Assess the quality of staining and initiates corrective action.
- Identify normal cellular elements on a stained blood smear.
- Use and maintain a bright field microscope.
- Differentiate between clinically significant and insignificant findings.
- Assess results, identify sources of interference, and initiate corrective action and/or follow up testing.
- Correlate peripheral blood smear findings with the CBC parameters and patient diagnosis.
CSMLS Competency Profile References
- Category 2: Data specimen collection and handling – 2.07-2.08; 2.11-2.12
- Category 3: Analytical processes – 3.01 – 3.02.01; 3.09.01 – 3.09.02; 3.13; 3.14 – 3.14.02
- Category 4: Interpretation and reporting results – 4.01-4.06
- Category 5: Quality management – 5.02; 5.05; 5.08; 5.10
- Category 6: Critical thinking – 6.04-6.06
Principle
Valuable information regarding a patients’ health can be obtained when all three cell lines are evaluated on a peripheral blood smear. “More can be learned from this test than many other routinely performed hematologic tests.” [1]The morphology of all three cell lines can be evaluated together for abnormalities that will be used by a physician to help with a patients’ diagnosis. WBC and PLT can be estimated and WBC cells can be differentiated into their different cell types.
The relationship between the blood smear and associated parameters generated via an automated blood analyzer (complete blood count – CBC), is important in the overall interpretation on the patients’ results, and correlation to hematologic diseases.
A stained blood smear is placed on microscope stage. The slide is initially scanned for cellular distribution and abnormalities using a 10x objective. After identifying a good morphology area, a WBC differential is performed (if ordered), followed by an evaluation of the RBC, RBC inclusions and PLTS.
Materials
- Microscope
- Immersion oil
Sample Type
- Properly made and well-stained blood smear.
Safety Consideration
Refer to MSD sheets for safety considerations located in the reagent prep room.
All specimens received in the lab must be regarded as potentially infectious so follow safe practices.
Refer to regulations and safety precautions in the student lab manual located in each student lab.
White Blood Cell (WBC) Differential
- Place the stained blood smear on the microscope stage and focus using the 10x objective. Scan the smear for staining quality, cellular distribution, abnormal white blood cell populations, platelets clumping and fibrin. Make sure you scan the edges and feather edge, as well as the body of the smear. Note: Fibrin indicates a clotted sample, and the test must be cancelled, and a recollect suggested.
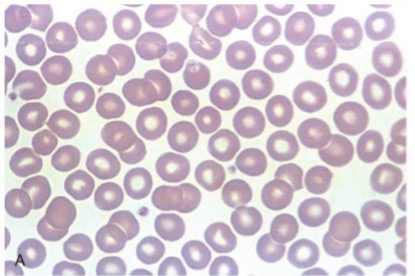
- Move the 40x objective in place and locate a good “morphology zone”, an area where the RBC’s are not quite touching or overlapping. Example of morphology zone on a stained peripheral blood smear.
- Place a drop of immersion oil on the slide and move the 50x objective into place. You may need to use the fine focus to bring the field of vision into focus.[2]
- The WBC differential is performed in a systemic manner using an “battlement” search pattern until 100 consecutive WBC are counted, using a cell counter. Refer to appendix 1 for example of a cell counter.

Example of counting technique. Credit:Keohane, E. M., Smith, L. J., & Walenga, J. M. (2016). Rodak’s hematology: Clinical principles and application (5th ed.) Elsevier. - Find an area just off center and start moving towards the thick area or out towards the feather edge.
- As the red blood cells start over lapping, move down/up one field and then proceed back towards the other edge of the slide.
- When the red blood cells start separating too much then move the microscope stage up/down one field and start back in the opposite direction. Make sure you continue moving in the same direction to avoid re-counting the same cells again.
- Continuing to follow this pattern until 100 cells have been counted and differentiated, making sure you count all WBC’s seen along the pathway.
- If you are having difficulty identifying some cells under 50X, you can use the 100X objective to confirm the cell, and then continue the count on the 50X objective.
- If you count less or more than 100 cells, you will have to perform the differential again.
- After counting 100 consecutive WBC’s, document the percentage of each cell type identified on the CBC/differential worksheet.
Red Blood Cell (RBC) Morphology
Stay within the morphology zone (an area where the RBC’s are not quite touching or overlapping) to complete the RBC examination. Using the 100x objective scan a minimum of 10 high power fields to determine the overall percentage of abnormal RBC’s present, using the following grading scheme.
- Abnormalities significant in large numbers includes:
- Target cells
- Ovalocytes
- Elliptocytes
- Stomatocytes
- Acanthocytes
- Burr cells
| If the overall average of the RBC abnormalities is between 10-30%, report as: | Moderate (2+) |
| If the overall average of the RBC abnormalities is >30%, report as: | Marked (3+) |
- Abnormalities significant in smaller numbers includes:
- Spherocytes
- Schistocytes (RBC fragments (Helmet cells, Blister cells, and keratocytes)
- Sickle cells
- Teardrop cells
| If the overall average of the RBC abnormalities is between 1 – 5%, report as: | Slight (1+) |
| If the overall average of the RBC abnormalities is between 6 – 20%, report as: | Moderate (2+) |
| If the overall average of the RBC abnormalities is > 20%, report as: | Marked (3+) |
- When reporting Polychromasia, use the following grading scheme:
| If the overall average of the RBC abnormalities is between 5 – 20%, report as: | Moderate (2+) |
| If the overall average of the RBC abnormalities is > 20%, report as: | Marked (3+) |
- Indicate present if you see any of the following RBC abnormalities on the blood smear:
- Dimorphism – Dual populations of RBC’s present
- Rouleaux formation
- RBC agglutination
- RBC inclusions including;
- Pappenheimer Bodies
- Basophilic Stippling
- Howell-Jolly Bodies
- Cabot Rings
- Hemoglobin Crystals
- Following RBC grading, document your findings on the worksheet provided.Platelet Morphology
- Using the 100x objective scan the smear and examine the platelets.
- When assessing the platelets, we evaluate the size, granularity and shape of the platelets.
Comment on the following if seen during your scan:
- Giant platelets present – Typically these are larger than a red blood cell.
- Hypogranular platelets present – Exhibit very little to no granules as seen in normal platelets
- If the platelets are clumped or exhibits satellitism, report the platelets as clumped. Clumping may cause a false decrease in the automated count, so you may need to remove the count from the CBC result.
- Any abnormal platelet morphology must be documented accordingly. They are not graded like red cell morphology. Only a comment indicating their presence.
Example
- Large or giant platelets present or seen.
- Hypogranular platelets present or seen.
- Irregular platelet shape should be noted, especially if their presence is in significant amounts.
- If it is determined that the platelets are clumped, report as follows:
- Clumped, but overall appear normal in numbers, then report as “clumped, appears normal on the smear”.
- Clumped, but overall appear increased in numbers, then report as “clumped, appears increased on the smear”.
- Clumped, but overall appear decreased in numbers, then report as “clumped, appears decreased on the smear”.
- Document your findings on the worksheet provided.
Procedural Notes
- If the total WBC count falls between 0.5 and 1.0 x109/L, a buffy coat preparation will be required in order to better evaluate the white blood cells.
- If the total WBC count is below 0.5 x109/L a WBC differential is NOT performed, but the platelets and red blood cell cells can still be evaluated.
- Under normal circumstances the manual WBC differential counts correlates well with the automated results. The presence of malignant cells or different stages of cellular development do not correlate well with the automated counts.
- If you are having difficulty identifying some WBC’s under 50X, you can use the 100X objective to confirm and then continue the count on the 50X objective.
- If you report RBC agglutination, you must incubate an aliquot of the patients’ blood at 37oC for about 15-20 minutes and rerun the specimen warm.
- You will also be required to make a pre-incubated and post-incubated blood smear for evaluation.
- Report RBC agglutination present on the pre-incubated slide
- Report the Platelet morphology and WBC differential from the pre-incubated slide
- Report the RBC morphology from the post-incubated slide
- Other abnormal RBC morphology may cause changes in the RBC parameters, so correlation between the CBC report and peripheral smear results is essential to assess.
- Any abnormal platelets, as indicated above, must be identified and commented on as per lab protocol.
Quality
- Peripheral blood smears are usually kept for a minimum of 2 years and must be stained, cover slipped and filed, as per lab protocol.
- WBC/platelet estimates aids in validating the counts from the hematology analyzer.
- Based on laboratory protocol, the senior technologist randomly picks previously completed peripheral smears to be re-evaluated for QC purposes. This helps to ensure consistency amongst the staff when reading smears.
- External QC (proficiency testing) can be achieved via an outside organization, such as the Canadian association of pathologists (CAP). Labs must apply to be a part of this process. Slides are sent out to the participating laboratories and staff reviews the slides, answers the questions provided and send the results back to CAP for review.
Interpretation
Depended on the disease present. Refer to the different hematologic diseases for interpretation.
Sources of Error
- Mislabeled blood smear will lead to incorrect patient results.
- Poorly made or stained smear may lead to erroneous results. Refer to Method 1 (making a blood smear) and method 2 (staining a blood smear) for potential sources of error.
