CSF Hemocytometer procedure
Cerebral Spinal Fluid (Csf) Analysis: Specimen Processing And Cell Count Procedure Using The Cyto C-Chip Disposable Hemocytometer
Learning Objectives
Provide proper instructions for processing a CSF specimen in the lab and performing manual cell counts and primary differentials on a Cerebral Spinal Fluid (CSF).
- Describe the processing of a CSF in the lab.
- Calculate RBC and WBC counts.
- Identify sources of error.
- Interpret the results.
Examples
- Category 2: Data specimen collection and handling – 2.01; 2.07-2.08; 2.11; 2.12
- Category 3: Analytical processes – 3.01; 3.09.01 – 3.09.02; 3.13
- Category 4: Interpretation and reporting results – 4.01-4.06
- Category 5: Quality management – 5.02; 5.05; 5.07
- Category 6: Critical thinking – 6.04-6.06
Principle
A CSF is normally clear and colorless in appearance, but an elevated WBC count (>200uL) or RBC count (> 400uL), bacteria or increased protein levels will result in a turbid and/or colored appearance. CSF is collected in 3 to 4 sterile non-anticoagulated plastic tubes and sent to the lab for evaluation. Following visual inspection of the tubes they are distributed to the appropriate benches for testing. In Hematology, a hemocytometer is used to numerate the RBC’s and WBCs, and if required, cytospin slides are prepared via cyto-centrifugation and evaluated for cell type and morphology.
Materials
- Filtered 2% acetic acid with new methylene blue stain (Refer to appendix 4 for preparation)
- Disposable Neubauer Hemacytometer. (Refer to appendix #1 for use)
- Light microscope
- Petri plate with moist gauze
- Cell counters
- 12 x 75 glass tubes
- Gauze/Kim wipes
- Eppendorf pipettes
- Timers
Sample Type
- Cerebral spinal fluid collected in 3 to 4 sterile non-anticoagulated graduated plastic tubes.
- A min of 2 to 4 mL in each of the tubes collected.
Safety Considerations
- The 2% acetic acid is considered TOXIC AND CORROSIVE!
- Refer to MSD sheets for safety considerations, located in the reagent prep room.
- All specimens received in the lab must be regarded as potentially infectious so follow safe practices.
- Refer to regulations and safety precautions in the student lab manual located in each student lab.
RECEIVING CSF SPECIMENS IN THE LAB
- Review requisition and confirm name, DOB and MRN match on all four tube labels
- Macroscopically inspect all four tubes and document the volume, clarity, and color of the numbered specimens on the worksheet for reporting in LIS.
- Distribute tubes to appropriate bench for testing:
- Chemistry: glucose, protein, LD, xanthochromia (may need to centrifuge to determine)
- Microbiology: Gram stain, C&S
- Hematology: WBC and RBC cell count, WBC differential
- Other testing: Cytology, molecular, flow cytometry, etc. If no fourth tube is received, you can use any other tube after testing is completed.
NOTE: If a small volume arrives, call the physician to prioritize testing.
Some labs may adjust the order of tubes for bench testing. Follow your SOP at your clinical site.
CELL COUNT PROCEDURE
- Prepare the hemocytometer for testing (Refer to appendix 1).
- Label the hemocytometer with patients’ information (Last name, first initial, and MRN #).
- Mix the CSF using gentle inversion until thoroughly mixed. (about 8-10 times).
- Pipette 10uL of “acid” treated CSF onto side A of the hemocytometer. To save time, this will be prepared in advance for the students. Note: RBCs are lysed leaving the WBC’s when treated with acid.
- Pipette 10uL of “neat” CSF onto the side B of the hemocytometer. Note: Both WBCs and RBCs are counted on the nest side.
- Male note of which side is neat and acid.
- Allow the hemocytometer to sit for 2 minutes before reading. This is important to allow the cells to settle out on the hemocytometer.
- When ready to count, place the hemacytometer on the microscope stage. You may have to adjust the microscope lighting to see the cells on the grid clearly.
- Note: Some microscopes do not have an adjustable condenser lens.
- Using a 10x objective, focus on the “Neat” side of the hemocytometer, and count all the cells in all nine squares. Document the results on the worksheet.
- Move over to the “Acid” side and count the cells in all nine squares. Document the results on the worksheet.
- Refer to Interpretation section below for calculating the WBC/RBC counts and document the results on the worksheet.
- Document the results on the worksheet.
Procedural Note
- Although the acetic acid is in a dilute form, avoid breathing in any fumes.
- Always keep the acetic acid covered when not using it.
- Ensure that the hemacytometer is free of dust and dirt before using.
- If there is going to be a delay in counting you need to keep the chamber in a moist environment to avoid drying out. You can moisten gauze in a petri dish and keep the hemacytometer there until you are ready to count the cells.
- Count the entire surface (all nine large squares) following the counting protocol indicated in the quality section.
- Perform the procedure in a biological safety cabinet.
- Note: We are using non biological hazardous material to prep the CSF specimen, so no need to set up in a biological safety cabinet.
QUALITY CONTROL
- Slides for evaluation of cellular morphology must be made ASAP to avoid cellular degradation and maintain cellular integrity.
- Ensure all samples are properly labelled and match accurately with the requisition.
- Tubes should contain about 2-4 mL of CSF and will be labelled in the numerical order in which they are collected. Note: volume will vary.
- Perform the hematology examination within 1 hour of collection, as per CLSI guidelines.
- Results for both WBCs and RBCs are reported in x 106/L.
- To get the total number of RBCs counted, subtract the acid side from the neat side.
- The total number of WBCs counted is the total of all cells counted on the acid side.
- Only count the cells that touch the top and left side of the squares to maintain consistency in your count. (Refer to appendix 3).
EXPECTED VALUES
| Appearance | Clear, colourless, and watery |
| RBC count | <1ul or 1.0 x 106/L |
| WBC count Adults | 0 – 5uL or 0 – 5 x106/L |
| WBC count Infants | 0 – 30uL or 0 – 30 x 106/L |
| Critical WBC value | > or = 10 x 106/L |
INTERPRETATION
Formula
- Number of cells x dilution divided by the volume. With CSF, no dilutions are done unless indicated.
Example
- A total of 16 cells counted on the acid side and a total of 25 cells counted on the neat side.
- Calculate the WBC and RBC counts.
- 25-16 = 9 – therefore there are 16 WBC and 9 RBC
- Note: 9 Large Squares counted (entire surface) = volume is 0.9
- 16 (WBC) /0.9(volume) 9(RBC)/0.9(volume)
- WBC =17.7 x 106/L RBC = 10 x 106/L
Note: both these values are high!
- Refer to Hemocytometer measurements
SOURCES OF ERROR
- Dust or dirt in the counting chamber can result in erroneous results.
- Contaminated diluting fluid may cause erroneous results.
- A high WBC count may make it difficult to obtain a reasonable count, so a secondary dilution may be required. Note: use the lowest possible dilution.
- You can run the CSF on the analyzer to obtain a RBC count, if present in excessive amounts on the hemocytometer. Follow lab protocol.
- Not maintaining a consistent counting routine may lead to erroneous results.
- Not allowing the chamber to settle before counting will result in erroneous results.
- Using the wrong tube for testing may lead to erroneous results. This may apply more to microbiology.
How to Use a Hemacytometer
Preparing the Hemocytometer.
- Remove the disposable hemocytometer from the package.
- Inspect it to make sure there are no cracks or dirt, etc.
- Label the Hemocytometer with patient name (last name, first initial) and the MRN number.
- Fill both sides of the Hemocytometer with the specimen/dilution.
- The entire surface of the chamber is counted for CSF specimens (all nine squares).
- Once the count is completed, discard the hemocytometer in the biohazard waste container.
Hemocytometer Measurements

- With CSF all nine squares are counted.
- For other fluids and EDTA specimens: White blood cells are counted in the four corner squares marked with a “W,” using a 10x low power objective.
- Platelets, not shown here, are counted in the entire center square (all twenty-five small squares), using a 40x dry objective. Not practice anymore.
- RBC counts are no longer performed manually.
Solid Circles are Counted, and the Open Circles are Not Counted
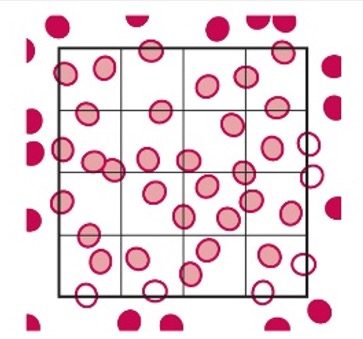
- This is a common counting strategy used in many labs to ensure accuracy in counting WBCs Count cells touching the top and left, but not the ones touching the right or bottom.
- Note: This only applies to the outside borders of the nine squares, not all squares.
Procedure for Preparation of 2% Acetic Acid with Methylene Blue
- Pour 490 mL of the Distilled Water into a 500mL cylinder.
- Carefully add 10mL of glacial acetic acid to the distilled water, bringin the total volume to 500mL.
- Cover with the parafilm and mix thoroughly.
- Add a very small amount of New Methylene blue powder to color the solution a very pale blue. The tip of a wooden applicator stick is suffient.
- Label a glass flask/bottle with appropriate safety information
- Filter the solution into a labelled glass container.
- Refrigerate the reagent until required.
